Dominant epitopes presented by prevalent HLA alleles permit wide use of banked CMVpp65 T cells in adoptive therapy
- PMID: 35605246
- PMCID: PMC9631666
- DOI: 10.1182/bloodadvances.2022007005
Dominant epitopes presented by prevalent HLA alleles permit wide use of banked CMVpp65 T cells in adoptive therapy
Abstract
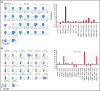
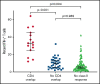
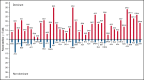
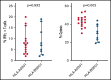
We established and characterized a bank of 138 CMVpp65 peptide-specific T-cell (CMVpp65CTLs) lines from healthy marrow transplant donors who consented to their use for treatment of individuals other than their transplant recipient. CMVpp65CTL lines included 131 containing predominantly CD8+ T cells and 7 CD4+ T cells. CD8+ CMVpp65CTLs were specific for 1 to 3 epitopes each presented by one of only 34 of the 148 class I alleles in the bank. Similarly, the 7 predominantly CD4+ CMVpp65CTL lines were each specific for epitopes presented by 14 of 40 HLA DR alleles in the bank. Although the number of HLA alleles presenting CMV epitopes is low, their prevalence is high, permitting selection of CMVpp65CTLs restricted by an HLA allele shared by transplant recipient and hematopoietic cell transplant donor for >90% of an ethnogeographically diverse population of hematopoietic cell transplant recipients. Within individuals, responses to CMVpp65 peptides presented by different HLA alleles are hierarchical. Furthermore, within groups, epitopes presented by HLA B*07:02 and HLA A*02:01 consistently elicit immunodominant CMVpp65CTLs, irrespective of other HLA alleles inherited. All dominant CMVpp65CTLs exhibited HLA-restricted cytotoxicity against epitope loaded targets and usually cleared CMV infections. However, immunodominant CMVpp65CTLs responding to epitopes presented by certain HLA B*35 alleles were ineffective in lysing CMV-infected cells in vitro or controlling CMV infections post adoptive therapy. Analysis of the hierarchy of T-cell responses to CMVpp65, the HLA alleles presenting immunodominant CMVpp65 epitopes, and the responses they induce may lead to detailed algorithms for optimal choice of third-party CMVpp65CTLs for effective adoptive therapy.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures



Similar articles
-
Third-party cytomegalovirus-specific T cells improved survival in refractory cytomegalovirus viremia after hematopoietic transplant.J Clin Invest. 2023 May 15;133(10):e165476. doi: 10.1172/JCI165476. J Clin Invest. 2023. PMID: 36951958 Free PMC article.
-
Immunotherapy with Donor T Cells Sensitized with Overlapping Pentadecapeptides for Treatment of Persistent Cytomegalovirus Infection or Viremia.Biol Blood Marrow Transplant. 2015 Sep;21(9):1663-78. doi: 10.1016/j.bbmt.2015.05.015. Epub 2015 May 29. Biol Blood Marrow Transplant. 2015. PMID: 26028505 Free PMC article. Clinical Trial.
-
Evaluation of suitable target antigens and immunoassays for high-accuracy immune monitoring of cytomegalovirus and Epstein-Barr virus-specific T cells as targets of interest in immunotherapeutic approaches.J Immunol Methods. 2014 Jun;408:101-13. doi: 10.1016/j.jim.2014.05.011. Epub 2014 May 28. J Immunol Methods. 2014. PMID: 24877879
-
Artificial antigen presenting cells that express prevalent HLA alleles: A step towards the broad application of antigen-specific adoptive cell therapies.Discov Med. 2009 Dec;8(43):210-8. Discov Med. 2009. PMID: 20040272 Review.
-
Refining human T-cell immunotherapy of cytomegalovirus disease: a mouse model with 'humanized' antigen presentation as a new preclinical study tool.Med Microbiol Immunol. 2016 Dec;205(6):549-561. doi: 10.1007/s00430-016-0471-0. Epub 2016 Aug 18. Med Microbiol Immunol. 2016. PMID: 27539576 Review.
Cited by
-
TCR sequencing: applications in immuno-oncology research.Immunooncol Technol. 2023 Feb 4;17:100373. doi: 10.1016/j.iotech.2023.100373. eCollection 2023 Mar. Immunooncol Technol. 2023. PMID: 36908996 Free PMC article. Review.
-
Third-party cytomegalovirus-specific T cells improved survival in refractory cytomegalovirus viremia after hematopoietic transplant.J Clin Invest. 2023 May 15;133(10):e165476. doi: 10.1172/JCI165476. J Clin Invest. 2023. PMID: 36951958 Free PMC article.
-
Characteristics of Norovirus capsid protein-specific CD8+ T-Cell responses in previously infected individuals.Virulence. 2024 Dec;15(1):2360133. doi: 10.1080/21505594.2024.2360133. Epub 2024 May 29. Virulence. 2024. PMID: 38803081 Free PMC article.
References
-
- Almyroudis NG, Jakubowski A, Jaffe D, et al. . Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2007;9(4):286-294. - PubMed
-
- Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83(7):1971-1979. - PubMed
-
- Reusser P, Einsele H, Lee J, et al. ; Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation . Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood. 2002;99(4):1159-1164. - PubMed
-
- Reusser P, Cordonnier C, Einsele H, et al. ; Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation (EBMT) . European survey of herpesvirus resistance to antiviral drugs in bone marrow transplant recipients. Bone Marrow Transplant. 1996;17(5):813-817. - PubMed
-
- Winston DJ, Ho WG, Bartoni K, et al. . Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993;118(3):179-184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

