Epigenetic ageing of the prefrontal cortex and cerebellum in humans and chimpanzees
- PMID: 35603816
- PMCID: PMC9621016
- DOI: 10.1080/15592294.2022.2080993
Epigenetic ageing of the prefrontal cortex and cerebellum in humans and chimpanzees
Abstract
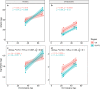
Epigenetic age has emerged as an important biomarker of biological ageing. It has revealed that some tissues age faster than others, which is vital to understanding the complex phenomenon of ageing and developing effective interventions. Previous studies have demonstrated that humans exhibit heterogeneity in pace of epigenetic ageing among brain structures that are consistent with differences in structural and microanatomical deterioration. Here, we add comparative data on epigenetic brain ageing for chimpanzees, humans' closest relatives. Such comparisons can further our understanding of which aspects of human ageing are evolutionarily conserved or specific to our species, especially given that humans are distinguished by a long lifespan, large brain, and, potentially, more severe neurodegeneration with age. Specifically, we investigated epigenetic ageing of the dorsolateral prefrontal cortex and cerebellum, of humans and chimpanzees by generating genome-wide CpG methylation data and applying established epigenetic clock algorithms to produce estimates of biological age for these tissues. We found that both species exhibit relatively slow epigenetic ageing in the brain relative to blood. Between brain structures, humans show a faster rate of epigenetic ageing in the dorsolateral prefrontal cortex compared to the cerebellum, which is consistent with previous findings. Chimpanzees, in contrast, show comparable rates of epigenetic ageing in the two brain structures. Greater epigenetic change in the human dorsolateral prefrontal cortex compared to the cerebellum may reflect both the protracted development of this structure in humans and its greater age-related vulnerability to neurodegenerative pathology.
Keywords: Alzheimer‘s disease; Methylation; Pan troglodytes; ageing; development; neuroscience.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

Similar articles
-
Comparative analysis reveals distinctive epigenetic features of the human cerebellum.PLoS Genet. 2021 May 6;17(5):e1009506. doi: 10.1371/journal.pgen.1009506. eCollection 2021 May. PLoS Genet. 2021. PMID: 33956822 Free PMC article.
-
Age-associated epigenetic change in chimpanzees and humans.Philos Trans R Soc Lond B Biol Sci. 2020 Nov 9;375(1811):20190616. doi: 10.1098/rstb.2019.0616. Epub 2020 Sep 21. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32951551 Free PMC article.
-
Insights into ageing rates comparison across tissues from recalibrating cerebellum DNA methylation clock.Geroscience. 2024 Feb;46(1):39-56. doi: 10.1007/s11357-023-00871-w. Epub 2023 Aug 19. Geroscience. 2024. PMID: 37597113 Free PMC article.
-
DNA Methylation as a Biomarker of Aging in Epidemiologic Studies.Methods Mol Biol. 2018;1856:219-231. doi: 10.1007/978-1-4939-8751-1_12. Methods Mol Biol. 2018. PMID: 30178254 Review.
-
The epigenetic clock as a predictor of disease and mortality risk: a systematic review and meta-analysis.Clin Epigenetics. 2019 Apr 11;11(1):62. doi: 10.1186/s13148-019-0656-7. Clin Epigenetics. 2019. PMID: 30975202 Free PMC article. Review.
Cited by
-
Vasopressin, and not oxytocin, receptor gene methylation is associated with individual differences in receptive joint attention in chimpanzees (Pan troglodytes).Autism Res. 2023 Apr;16(4):713-722. doi: 10.1002/aur.2895. Epub 2023 Feb 4. Autism Res. 2023. PMID: 36738470 Free PMC article.
-
Quantification of cytosine modifications in the aged mouse brain.Neuropsychopharmacol Rep. 2024 Mar;44(1):250-255. doi: 10.1002/npr2.12396. Epub 2023 Dec 6. Neuropsychopharmacol Rep. 2024. PMID: 38058257 Free PMC article.
-
Brain aging and Alzheimer's disease, a perspective from non-human primates.Aging (Albany NY). 2024 Oct 29;16(20):13145-13171. doi: 10.18632/aging.206143. Epub 2024 Oct 29. Aging (Albany NY). 2024. PMID: 39475348 Free PMC article. Review.
-
Early life adversities and lifelong health outcomes: A review of the literature on large, social, long-lived nonhuman mammals.Neurosci Biobehav Rev. 2023 Sep;152:105297. doi: 10.1016/j.neubiorev.2023.105297. Epub 2023 Jun 28. Neurosci Biobehav Rev. 2023. PMID: 37391110 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
