Neutrophil Extracellular Traps Regulate HMGB1 Translocation and Kupffer Cell M1 Polarization During Acute Liver Transplantation Rejection
- PMID: 35603144
- PMCID: PMC9120840
- DOI: 10.3389/fimmu.2022.823511
Neutrophil Extracellular Traps Regulate HMGB1 Translocation and Kupffer Cell M1 Polarization During Acute Liver Transplantation Rejection
Abstract
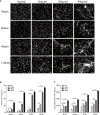
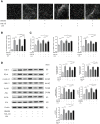
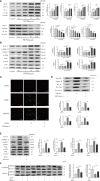
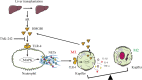
Neutrophil extracellular traps (NETs) play important roles in hepatic ischemic reperfusion injury (IRI) and acute rejection (AR)-induced immune responses to inflammation. After liver transplantation, HMGB1, an inflammatory mediator, contributes to the development of AR. Even though studies have found that HMGB1 can promote NET formation, the correlation between NETs and HMGB1 in the development of AR following liver transplantation has not been elucidated. In this study, levels of serum NETs were significantly elevated in patients after liver transplantation. Moreover, we found that circulating levels of NETs were negatively correlated with liver function. In addition, liver transplantation and elevated extracellular HMGB1 promoted NET formation. The HMGB1/TLR-4/MAPK signaling pathway, which is initiated by HMGB1, participates in NET processes. Moreover, in the liver, Kupffer cells were found to be the main cells secreting HMGB1. NETs induced Kupffer cell M1 polarization and decreased the intracellular translocation of HMGB1 by inhibiting DNase-1. Additionally, co-treatment with TAK-242 (a TLR-4 inhibitor) and rapamycin more effectively alleviated the damaging effects of AR following liver transplantation than either drug alone.
Keywords: Kupffer cell; acute rejection; high mobility group box-1 (HMGB1); liver transplantation; neutrophil extracellular traps (NETs).
Copyright © 2022 Liu, Pu, Qin, Gong, Huang, Luo, Mou, Zhou, Shen and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Salidroside ameliorates acute liver transplantation rejection in rats by inhibiting neutrophil extracellular trap formation.Acta Biochim Biophys Sin (Shanghai). 2024 Jun 25;56(6):833-843. doi: 10.3724/abbs.2024055. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38716542 Free PMC article.
-
Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury.Hepatology. 2015 Aug;62(2):600-14. doi: 10.1002/hep.27841. Epub 2015 May 29. Hepatology. 2015. PMID: 25855125 Free PMC article.
-
Neutrophil extracellular traps induced by the hypoxic microenvironment in gastric cancer augment tumour growth.Cell Commun Signal. 2023 May 1;21(1):86. doi: 10.1186/s12964-023-01112-5. Cell Commun Signal. 2023. PMID: 37127629 Free PMC article.
-
Role of Immuno-Inflammatory Signals in Liver Ischemia-Reperfusion Injury.Cells. 2022 Jul 17;11(14):2222. doi: 10.3390/cells11142222. Cells. 2022. PMID: 35883665 Free PMC article. Review.
-
Neutrophil extracellular traps and complications of liver transplantation.Front Immunol. 2022 Nov 17;13:1054753. doi: 10.3389/fimmu.2022.1054753. eCollection 2022. Front Immunol. 2022. PMID: 36466888 Free PMC article. Review.
Cited by
-
Identification of organs of origin of macrophages that produce presepsin via neutrophil extracellular trap phagocytosis.Sci Rep. 2024 Jul 16;14(1):16386. doi: 10.1038/s41598-024-66916-y. Sci Rep. 2024. PMID: 39013974 Free PMC article.
-
Inhibition of Toll-like Receptor 4 Using Small Molecule, TAK-242, Protects Islets from Innate Immune Responses.Cells. 2024 Feb 27;13(5):416. doi: 10.3390/cells13050416. Cells. 2024. PMID: 38474380 Free PMC article.
-
Analysis and experimental validation of IL-17 pathway and key genes as central roles associated with inflammation in hepatic ischemia-reperfusion injury.Sci Rep. 2024 Mar 18;14(1):6423. doi: 10.1038/s41598-024-57139-2. Sci Rep. 2024. PMID: 38494504 Free PMC article.
-
Neutrophil Extracellular Traps in Cardiovascular and Aortic Disease: A Narrative Review on Molecular Mechanisms and Therapeutic Targeting.Int J Mol Sci. 2024 Apr 3;25(7):3983. doi: 10.3390/ijms25073983. Int J Mol Sci. 2024. PMID: 38612791 Free PMC article. Review.
-
Global research hotspots and trends of acute rejection after liver transplantation from 1988 to 2022: a bibliometric analysis.Front Pharmacol. 2024 Apr 17;15:1357468. doi: 10.3389/fphar.2024.1357468. eCollection 2024. Front Pharmacol. 2024. PMID: 38694927 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

