Dual-Role Ubiquitination Regulation Shuttling the Entire Life Cycle of the Flaviviridae
- PMID: 35602051
- PMCID: PMC9120866
- DOI: 10.3389/fmicb.2022.835344
Dual-Role Ubiquitination Regulation Shuttling the Entire Life Cycle of the Flaviviridae
Abstract
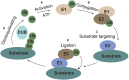
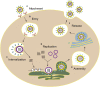
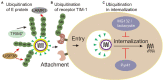
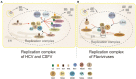
Ubiquitination is a reversible protein post-translational modification that regulates various pivotal physiological and pathological processes in all eukaryotes. Recently, the antiviral immune response is enhanced by the regulation of ubiquitination. Intriguingly, Flaviviridae viruses can ingeniously hijack the ubiquitination system to help them survive, which has become a hot topic among worldwide researchers. The Flaviviridae family members, such as HCV and CSFV, can cause serious diseases of humans and animals around the world. The multiple roles of ubiquitination involved in the life cycle of Flaviviridae family would open new sight for future development of antiviral tactic. Here, we discuss recent advances with regard to functional roles of ubiquitination and some ubiquitin-like modifications in the life cycle of Flaviviridae infection, shedding new light on the antiviral mechanism research and therapeutic drug development.
Keywords: CSFV; Flaviviridae; HCV; TRIM proteins; antiviral immunity; ubiquitin system.
Copyright © 2022 Cai, Liu, Tian, Fu, Yang, Chen, Zhang, Fang, Shen, Wang, Gou and Zuo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
To TRIM or not to TRIM: the balance of host-virus interactions mediated by the ubiquitin system.J Gen Virol. 2019 Dec;100(12):1641-1662. doi: 10.1099/jgv.0.001341. J Gen Virol. 2019. PMID: 31661051 Free PMC article.
-
Ubiquitination, Biotech Startups, and the Future of TRIM Family Proteins: A TRIM-Endous Opportunity.Cells. 2021 Apr 25;10(5):1015. doi: 10.3390/cells10051015. Cells. 2021. PMID: 33923045 Free PMC article. Review.
-
The Roles of Ubiquitination in Pathogenesis of Influenza Virus Infection.Int J Mol Sci. 2022 Apr 21;23(9):4593. doi: 10.3390/ijms23094593. Int J Mol Sci. 2022. PMID: 35562987 Free PMC article. Review.
-
The ESCRT-I Subunit Tsg101 Plays Novel Dual Roles in Entry and Replication of Classical Swine Fever Virus.J Virol. 2021 Feb 24;95(6):e01928-20. doi: 10.1128/JVI.01928-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33328308 Free PMC article.
-
The Small-Compound Inhibitor K22 Displays Broad Antiviral Activity against Different Members of the Family Flaviviridae and Offers Potential as a Panviral Inhibitor.Antimicrob Agents Chemother. 2018 Oct 24;62(11):e01206-18. doi: 10.1128/AAC.01206-18. Print 2018 Nov. Antimicrob Agents Chemother. 2018. PMID: 30181371 Free PMC article.
Cited by
-
A single-cell atlas of the Culex tarsalis midgut during West Nile virus infection.bioRxiv [Preprint]. 2024 Nov 24:2024.07.23.603613. doi: 10.1101/2024.07.23.603613. bioRxiv. 2024. PMID: 39091762 Free PMC article. Preprint.
-
Protein Quality Control Systems and ER Stress as Key Players in SARS-CoV-2-Induced Neurodegeneration.Cells. 2024 Jan 9;13(2):123. doi: 10.3390/cells13020123. Cells. 2024. PMID: 38247815 Free PMC article. Review.
-
The Dynamic Landscape of Capsid Proteins and Viral RNA Interactions in Flavivirus Genome Packaging and Virus Assembly.Pathogens. 2024 Jan 28;13(2):120. doi: 10.3390/pathogens13020120. Pathogens. 2024. PMID: 38392858 Free PMC article. Review.
References
-
- Assenberg R., Mastrangelo E., Walter T. S., Verma A., Milani M., Owens R. J., et al. . (2009). Crystal structure of a novel conformational state of the flavivirus NS3 protein: implications for polyprotein processing and viral replication. J. Virol. 83, 12895–12906. doi: 10.1128/JVI.00942-09, PMID: - DOI - PMC - PubMed
-
- Barouch-Bentov R., Neveu G., Xiao F., Beer M., Bekerman E., Schor S., et al. . (2016). Hepatitis C virus proteins interact with the endosomal sorting complex required for transport (ESCRT) machinery via ubiquitination to facilitate viral envelopment. MBio 7, e01456–e01516. doi: 10.1128/mBio.01456-16, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

