Serotonin signals through postsynaptic Gαq, Trio RhoGEF, and diacylglycerol to promote Caenorhabditis elegans egg-laying circuit activity and behavior
- PMID: 35579369
- PMCID: PMC9252285
- DOI: 10.1093/genetics/iyac084
Serotonin signals through postsynaptic Gαq, Trio RhoGEF, and diacylglycerol to promote Caenorhabditis elegans egg-laying circuit activity and behavior
Abstract
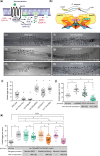
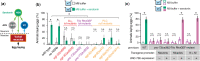
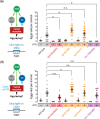
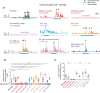
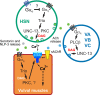
Activated Gαq signals through phospholipase-Cβ and Trio, a Rho GTPase exchange factor (RhoGEF), but how these distinct effector pathways promote cellular responses to neurotransmitters like serotonin remains poorly understood. We used the egg-laying behavior circuit of Caenorhabditis elegans to determine whether phospholipase-Cβ and Trio mediate serotonin and Gαq signaling through independent or related biochemical pathways. Our genetic rescue experiments suggest that phospholipase-Cβ functions in neurons while Trio Rho GTPase exchange factor functions in both neurons and the postsynaptic vulval muscles. While Gαq, phospholipase-Cβ, and Trio Rho GTPase exchange factor mutants fail to lay eggs in response to serotonin, optogenetic stimulation of the serotonin-releasing HSN neurons restores egg laying only in phospholipase-Cβ mutants. Phospholipase-Cβ mutants showed vulval muscle Ca2+ transients while strong Gαq and Trio Rho GTPase exchange factor mutants had little or no vulval muscle Ca2+ activity. Treatment with phorbol 12-myristate 13-acetate that mimics 1,2-diacylglycerol, a product of PIP2 hydrolysis, rescued egg-laying circuit activity and behavior defects of Gαq signaling mutants, suggesting both phospholipase-C and Rho signaling promote synaptic transmission and egg laying via modulation of 1,2-diacylglycerol levels. 1,2-Diacylglycerol activates effectors including UNC-13; however, we find that phorbol esters, but not serotonin, stimulate egg laying in unc-13 and phospholipase-Cβ mutants. These results support a model where serotonin signaling through Gαq, phospholipase-Cβ, and UNC-13 promotes neurotransmitter release, and that serotonin also signals through Gαq, Trio Rho GTPase exchange factor, and an unidentified, phorbol 12-myristate 13-acetate-responsive effector to promote postsynaptic muscle excitability. Thus, the same neuromodulator serotonin can signal in distinct cells and effector pathways to coordinate activation of a motor behavior circuit.
Keywords: Caenorhabditis elegans; DAG; G protein; Trio RhoGEF; calcium imaging; circuit activity; neurotransmission; optogenetics; serotonin; synapse.
© The Author(s) 2022. Published by Oxford University Press on behalf of Genetics Society of America. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
The Sex-Specific VC Neurons Are Mechanically Activated Motor Neurons That Facilitate Serotonin-Induced Egg Laying in C. elegans.J Neurosci. 2021 Apr 21;41(16):3635-3650. doi: 10.1523/JNEUROSCI.2150-20.2021. Epub 2021 Mar 9. J Neurosci. 2021. PMID: 33687965 Free PMC article.
-
Homeostatic Feedback Modulates the Development of Two-State Patterned Activity in a Model Serotonin Motor Circuit in Caenorhabditis elegans.J Neurosci. 2018 Jul 11;38(28):6283-6298. doi: 10.1523/JNEUROSCI.3658-17.2018. Epub 2018 Jun 11. J Neurosci. 2018. PMID: 29891728 Free PMC article.
-
Trio's Rho-specific GEF domain is the missing Galpha q effector in C. elegans.Genes Dev. 2007 Nov 1;21(21):2731-46. doi: 10.1101/gad.1592007. Epub 2007 Oct 17. Genes Dev. 2007. PMID: 17942708 Free PMC article.
-
Neurotransmitter signaling through heterotrimeric G proteins: insights from studies in C. elegans.WormBook. 2018 Dec 11;2018:1-52. doi: 10.1895/wormbook.1.75.2. WormBook. 2018. PMID: 26937633 Free PMC article. Review.
-
Function and regulation of the Rho guanine nucleotide exchange factor Trio.Small GTPases. 2014;5:e29769. doi: 10.4161/sgtp.29769. Epub 2014 Jul 2. Small GTPases. 2014. PMID: 24987837 Free PMC article. Review.
Cited by
-
Muscle-directed mechanosensory feedback activates egg-laying circuit activity and behavior in Caenorhabditis elegans.Curr Biol. 2023 Jun 5;33(11):2330-2339.e8. doi: 10.1016/j.cub.2023.05.008. Epub 2023 May 25. Curr Biol. 2023. PMID: 37236183 Free PMC article.
-
NALCN Channels Are Not Major targets of Gα o or Gα q Modulation in the C. elegans Egg-Laying Behavior Circuit.MicroPubl Biol. 2024 Jan 11;2024:10.17912/micropub.biology.001065. doi: 10.17912/micropub.biology.001065. eCollection 2024. MicroPubl Biol. 2024. PMID: 38287929 Free PMC article.
-
Multiple Subthreshold GPCR Signals Combined by the G-Proteins Gαq and Gαs Activate the Caenorhabditis elegans Egg-Laying Muscles.J Neurosci. 2023 May 24;43(21):3789-3806. doi: 10.1523/JNEUROSCI.2301-22.2023. Epub 2023 Apr 13. J Neurosci. 2023. PMID: 37055179 Free PMC article.
References
-
- Ananthanarayanan B, Stahelin RV, Digman MA, Cho W.. Activation mechanisms of conventional protein kinase C isoforms are determined by the ligand affinity and conformational flexibility of their C1 domains. J Biol Chem. 2003;278(47):46886–46894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

