An imidazole modified lipid confers enhanced mRNA-LNP stability and strong immunization properties in mice and non-human primates
- PMID: 35576809
- PMCID: PMC9078044
- DOI: 10.1016/j.biomaterials.2022.121570
An imidazole modified lipid confers enhanced mRNA-LNP stability and strong immunization properties in mice and non-human primates
Abstract
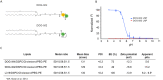
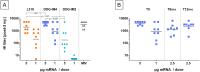
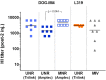
The mRNA vaccine technology has promising applications to fight infectious diseases as demonstrated by the licensing of two mRNA-based vaccines, Comirnaty® (Pfizer/BioNtech) and Spikevax® (Moderna), in the context of the Covid-19 crisis. Safe and effective delivery systems are essential to the performance of these vaccines and lipid nanoparticles (LNPs) able to entrap, protect and deliver the mRNA in vivo are considered by many as the current "best in class". Nevertheless, current mRNA/LNP vaccine technology has still some limitations, one of them being thermostability, as evidenced by the ultracold distribution chain required for the licensed vaccines. We found that the thermostability of mRNA/LNP, could be improved by a novel imidazole modified lipid, DOG-IM4, in combination with standard helper lipids. DOG-IM4 comprises an ionizable head group consisting of imidazole, a dioleoyl lipid tail and a short flexible polyoxyethylene spacer between the head and tail. Here we describe the synthesis of DOG-IM4 and show that DOG-IM4 LNPs confer strong immunization properties to influenza HA mRNA in mice and macaques and a remarkable stability to the encapsulated mRNA when stored liquid in phosphate buffered saline at 4 °C. We speculate the increased stability to result from some specific attributes of the lipid's imidazole head group.
Keywords: Imidazole lipid; Ionizable lipid; Lipid nanoparticles; Storage stability; mRNA vaccine.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Chemistry of Lipid Nanoparticles for RNA Delivery.Acc Chem Res. 2022 Jan 4;55(1):2-12. doi: 10.1021/acs.accounts.1c00544. Epub 2021 Dec 1. Acc Chem Res. 2022. PMID: 34850635 Review.
-
Production and Evaluation of Nucleoside-Modified mRNA Vaccines for Infectious Diseases.Methods Mol Biol. 2024;2786:167-181. doi: 10.1007/978-1-0716-3770-8_7. Methods Mol Biol. 2024. PMID: 38814394
-
Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs.Drug Metab Pharmacokinet. 2021 Dec;41:100424. doi: 10.1016/j.dmpk.2021.100424. Epub 2021 Oct 10. Drug Metab Pharmacokinet. 2021. PMID: 34757287 Free PMC article. Review.
-
Enzyme-Catalyzed One-Step Synthesis of Ionizable Cationic Lipids for Lipid Nanoparticle-Based mRNA COVID-19 Vaccines.ACS Nano. 2022 Nov 22;16(11):18936-18950. doi: 10.1021/acsnano.2c07822. Epub 2022 Oct 21. ACS Nano. 2022. PMID: 36269150
-
From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases.Acta Biomater. 2021 Sep 1;131:16-40. doi: 10.1016/j.actbio.2021.06.023. Epub 2021 Jun 18. Acta Biomater. 2021. PMID: 34153512 Free PMC article. Review.
Cited by
-
Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics.Exp Mol Med. 2023 Oct;55(10):2085-2096. doi: 10.1038/s12276-023-01086-x. Epub 2023 Oct 2. Exp Mol Med. 2023. PMID: 37779140 Free PMC article. Review.
-
How far are the new wave of mRNA drugs from us? mRNA product current perspective and future development.Front Immunol. 2022 Sep 12;13:974433. doi: 10.3389/fimmu.2022.974433. eCollection 2022. Front Immunol. 2022. PMID: 36172353 Free PMC article. Review.
-
Continuous freeze-drying of messenger RNA lipid nanoparticles enables storage at higher temperatures.J Control Release. 2023 May;357:149-160. doi: 10.1016/j.jconrel.2023.03.039. Epub 2023 Mar 30. J Control Release. 2023. PMID: 36958400 Free PMC article.
-
A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability.NPJ Vaccines. 2022 Nov 2;7(1):136. doi: 10.1038/s41541-022-00549-y. NPJ Vaccines. 2022. PMID: 36323666 Free PMC article.
-
Chemistry and Art of Developing Lipid Nanoparticles for Biologics Delivery: Focus on Development and Scale-Up.Pharmaceutics. 2024 Jan 19;16(1):131. doi: 10.3390/pharmaceutics16010131. Pharmaceutics. 2024. PMID: 38276502 Free PMC article. Review.
References
-
- Thran M., Mukherjee J., Pönisch M., Fiedler K., Thess A., Mui B.L., Hope M.J., Tam Y.K., Horscroft N., Heidenreich R., Fotin-Mleczek M., Shoemaker C.B., Schlake T. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017;9:1434–1447. doi: 10.15252/emmm.201707678. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

