Contributions of the Catechol-O-Methyltransferase Val158Met Polymorphism to Changes in Brain Iron Across Adulthood and Their Relationships to Working Memory
- PMID: 35571998
- PMCID: PMC9091601
- DOI: 10.3389/fnhum.2022.838228
Contributions of the Catechol-O-Methyltransferase Val158Met Polymorphism to Changes in Brain Iron Across Adulthood and Their Relationships to Working Memory
Abstract
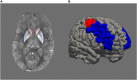
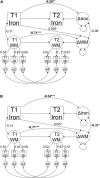
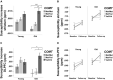
Ageing is associated with excessive free brain iron, which may induce oxidative stress and neuroinflammation, likely causing cognitive deficits. Lack of dopamine may be a factor behind the increase of iron with advancing age, as it has an important role in cellular iron homoeostasis. We investigated the effect of COMT Val 158 Met (rs4680), a polymorphism crucial for dopamine degradation and proxy for endogenous dopamine, on iron accumulation and working memory in a longitudinal lifespan sample (n = 208, age 20-79 at baseline, mean follow-up time = 2.75 years) using structural equation modelling. Approximation of iron content was assessed using quantitative susceptibility mapping in striatum and dorsolateral prefrontal cortex (DLPFC). Iron accumulated in both striatum and DLPFC during the follow-up period. Greater iron accumulation in DLPFC was associated with more deleterious change in working memory. Older (age 50-79) Val homozygotes (with presumably lower endogenous dopamine) accumulated more iron than older Met carriers in both striatum and DLPFC, no such differences were observed among younger adults (age 20-49). In conclusion, individual differences in genetic predisposition related to low dopamine levels increase iron accumulation, which in turn may trigger deleterious change in working memory. Future studies are needed to better understand how dopamine may modulate iron accumulation across the human lifespan.
Keywords: COMT; QSM; SEM; ageing; brain iron; dopamine; longitudinal; working memory.
Copyright © 2022 Gustavsson, Papenberg, Falahati, Laukka and Kalpouzos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The catechol-O-methyltransferase (COMT) Val158Met genotype modulates working memory-related dorsolateral prefrontal response and performance in bipolar disorder.Bipolar Disord. 2017 May;19(3):214-224. doi: 10.1111/bdi.12497. Epub 2017 May 23. Bipolar Disord. 2017. PMID: 28544426
-
COMT val158met polymorphism and molecular alterations in the human dorsolateral prefrontal cortex: Differences in controls and in schizophrenia.Schizophr Res. 2016 May;173(1-2):94-100. doi: 10.1016/j.schres.2016.03.019. Epub 2016 Mar 24. Schizophr Res. 2016. PMID: 27021555 Free PMC article.
-
Putative role of the COMT gene polymorphism (Val158Met) on verbal working memory functioning in a healthy population.Am J Med Genet B Neuropsychiatr Genet. 2008 Sep 5;147B(6):898-902. doi: 10.1002/ajmg.b.30705. Am J Med Genet B Neuropsychiatr Genet. 2008. PMID: 18213617
-
Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors.CNS Drugs. 2007;21(7):535-57. doi: 10.2165/00023210-200721070-00002. CNS Drugs. 2007. PMID: 17579498 Review.
-
Catechol-O-methyltransferase, dopamine, and sleep-wake regulation.Sleep Med Rev. 2015 Aug;22:47-53. doi: 10.1016/j.smrv.2014.10.006. Epub 2014 Oct 27. Sleep Med Rev. 2015. PMID: 25466290 Review.
Cited by
-
Quantitative susceptibility mapping of brain iron in healthy aging and cognition.Neuroimage. 2023 Nov 15;282:120401. doi: 10.1016/j.neuroimage.2023.120401. Epub 2023 Oct 5. Neuroimage. 2023. PMID: 37802405 Free PMC article. Review.
-
Dopamine D1-signaling modulates maintenance of functional network segregation in aging.Aging Brain. 2023 Jun 15;3:100079. doi: 10.1016/j.nbas.2023.100079. eCollection 2023. Aging Brain. 2023. PMID: 37408790 Free PMC article.
-
Exploring the links among brain iron accumulation, cognitive performance, and dietary intake in older adults: A longitudinal MRI study.Neurobiol Aging. 2025 Jan;145:1-12. doi: 10.1016/j.neurobiolaging.2024.10.006. Epub 2024 Oct 19. Neurobiol Aging. 2025. PMID: 39447489
-
Perturbed iron biology in the prefrontal cortex of people with schizophrenia.Mol Psychiatry. 2023 May;28(5):2058-2070. doi: 10.1038/s41380-023-01979-3. Epub 2023 Feb 7. Mol Psychiatry. 2023. PMID: 36750734 Free PMC article.
References
-
- Andersson J., Jenkinson M., Smith S. (2007). Non-Linear Registration aka Spatial Normalisation. FMRIB Technial Report TR07JA2. Oxford: Oxford University Press.
LinkOut - more resources
Full Text Sources
Miscellaneous

