HOXC6 Regulates the Epithelial-Mesenchymal Transition through the TGF- β/Smad Signaling Pathway and Predicts a Poor Prognosis in Glioblastoma
- PMID: 35571491
- PMCID: PMC9098331
- DOI: 10.1155/2022/8016102
HOXC6 Regulates the Epithelial-Mesenchymal Transition through the TGF- β/Smad Signaling Pathway and Predicts a Poor Prognosis in Glioblastoma
Abstract
Background: The HOX gene family of transcription factors, characterized by conserved homeodomains, is positively correlated with the resistance to chemotherapy drugs and poor prognosis, as well as the initiating potential of gliomas. However, there are few studies regarding the HOXC6 gene in glioma cells. Therefore, in the present study, we explored the regulatory roles and detailed mechanisms underlying the relationship between HOXC6 and the progression of GBM.
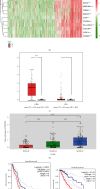
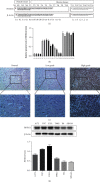
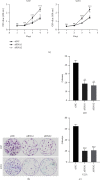
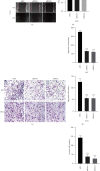
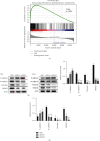
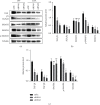
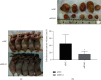
Methods: The expression levels and prognostic value of HOXC6 in GBM were evaluated using the data obtained from the GCCA, GEPIA, and ONCOMINE databases. The relationship between GBM prognosis and levels of HOXC6 was identified using Kaplan-Meier curves. The protein levels of HOXC6 in GBM and adjacent normal tissues were identified via Western blot and immunohistochemistry (IHC) staining methods. Lentiviruses containing full-length HOXC6 and HOXC6 specific siRNA sequences were used to overexpress and knock down, respectively, the expression of HOXC6 in U87 and U251 cells. The role of HOXC6 in the regulation of migration and proliferation of GBM cells was accessed using Transwell, wound healing, CCK-8, and colony formation assays. The activation of the TGF-β/Smad signaling pathway was detected via Western blotting.
Results: Compared to normal tissues and control cells, GBM tissues and cell lines showed higher expressions of HOXC6. The expression of HOXC6 was associated with disease-free and the overall survival of GBM patients. Additionally, positive correlations between the expression of HOXC6 and the migration and proliferation of GBM cells were observed in vitro. The mechanistic analyses indicated that HOXC6 exerts its promotive effect on the progression and invasion of glioma cells by promoting the activation of the EMT and TGF-β/Smad signaling pathways.
Conclusions: HOXC6 enhances the migration and proliferation of GBM by activating the EMT signaling pathway.
Copyright © 2022 Sun Eryi et al.
Conflict of interest statement
We have no conflicts of interest to disclose.
Figures
Similar articles
-
Overexpression of HOXC6 promotes cell proliferation and migration via MAPK signaling and predicts a poor prognosis in glioblastoma.Cancer Manag Res. 2019 Sep 3;11:8167-8179. doi: 10.2147/CMAR.S209904. eCollection 2019. Cancer Manag Res. 2019. PMID: 31564976 Free PMC article.
-
Knockdown of HOXC6 inhibits glioma cell proliferation and induces cell cycle arrest by targeting WIF-1 in vitro and vivo.Pathol Res Pract. 2018 Nov;214(11):1818-1824. doi: 10.1016/j.prp.2018.09.001. Epub 2018 Sep 12. Pathol Res Pract. 2018. PMID: 30228024
-
HOXC6 gene silencing inhibits epithelial-mesenchymal transition and cell viability through the TGF-β/smad signaling pathway in cervical carcinoma cells.Cancer Cell Int. 2018 Dec 12;18:204. doi: 10.1186/s12935-018-0680-2. eCollection 2018. Cancer Cell Int. 2018. PMID: 30559605 Free PMC article.
-
EMP3 as a prognostic biomarker correlates with EMT in GBM.BMC Cancer. 2024 Jan 17;24(1):89. doi: 10.1186/s12885-023-11796-0. BMC Cancer. 2024. PMID: 38229014 Free PMC article.
-
The PDK1/c‑Jun pathway activated by TGF‑β induces EMT and promotes proliferation and invasion in human glioblastoma.Int J Oncol. 2018 Nov;53(5):2067-2080. doi: 10.3892/ijo.2018.4525. Epub 2018 Aug 14. Int J Oncol. 2018. PMID: 30106127
Cited by
-
Predicting the prognosis of glioma patients with TERT promoter mutations and guiding the specific immune profile of immune checkpoint blockade therapy.Aging (Albany NY). 2024 Mar 18;16(6):5618-5633. doi: 10.18632/aging.205668. Epub 2024 Mar 18. Aging (Albany NY). 2024. PMID: 38499392 Free PMC article.
-
Role of homeobox genes in cancer: immune system interactions, long non-coding RNAs, and tumor progression.Mol Biol Rep. 2024 Sep 6;51(1):964. doi: 10.1007/s11033-024-09857-z. Mol Biol Rep. 2024. PMID: 39240390 Review.
References
LinkOut - more resources
Full Text Sources

