Proteostasis Perturbations and Their Roles in Causing Sterile Inflammation and Autoinflammatory Diseases
- PMID: 35563729
- PMCID: PMC9103147
- DOI: 10.3390/cells11091422
Proteostasis Perturbations and Their Roles in Causing Sterile Inflammation and Autoinflammatory Diseases
Abstract
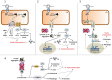
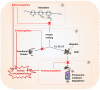
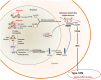
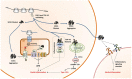
Proteostasis, a portmanteau of the words protein and homeostasis, refers to the ability of eukaryotic cells to maintain a stable proteome by acting on protein synthesis, quality control and/or degradation. Over the last two decades, an increasing number of disorders caused by proteostasis perturbations have been identified. Depending on their molecular etiology, such diseases may be classified into ribosomopathies, proteinopathies and proteasomopathies. Strikingly, most-if not all-of these syndromes exhibit an autoinflammatory component, implying a direct cause-and-effect relationship between proteostasis disruption and the initiation of innate immune responses. In this review, we provide a comprehensive overview of the molecular pathogenesis of these disorders and summarize current knowledge of the various mechanisms by which impaired proteostasis promotes autoinflammation. We particularly focus our discussion on the notion of how cells sense and integrate proteostasis perturbations as danger signals in the context of autoinflammatory diseases to provide insights into the complex and multiple facets of sterile inflammation.
Keywords: autoinflammation; proteasomopathies; proteinopathies; proteostasis; ribosomopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Dysfunction in protein clearance by the proteasome: impact on autoinflammatory diseases.Semin Immunopathol. 2015 Jul;37(4):323-33. doi: 10.1007/s00281-015-0486-4. Epub 2015 May 12. Semin Immunopathol. 2015. PMID: 25963519 Review.
-
New monogenic autoinflammatory diseases--a clinical overview.Semin Immunopathol. 2015 Jul;37(4):387-94. doi: 10.1007/s00281-015-0493-5. Epub 2015 May 12. Semin Immunopathol. 2015. PMID: 25963521 Free PMC article. Review.
-
Autoinflammatory Disorders: A Review and Update on Pathogenesis and Treatment.Am J Clin Dermatol. 2019 Aug;20(4):539-564. doi: 10.1007/s40257-019-00440-y. Am J Clin Dermatol. 2019. PMID: 30997665 Review.
-
New players driving inflammation in monogenic autoinflammatory diseases.Nat Rev Rheumatol. 2015 Jan;11(1):11-20. doi: 10.1038/nrrheum.2014.158. Epub 2014 Sep 23. Nat Rev Rheumatol. 2015. PMID: 25247411 Review.
-
Hereditary Autoinflammatory Disorders: Recognition and Treatment.Immunol Allergy Clin North Am. 2019 Feb;39(1):13-29. doi: 10.1016/j.iac.2018.08.004. Epub 2018 Nov 1. Immunol Allergy Clin North Am. 2019. PMID: 30466770 Review.
Cited by
-
PSMD11 loss-of-function variants correlate with a neurobehavioral phenotype, obesity, and increased interferon response.Am J Hum Genet. 2024 Jul 11;111(7):1352-1369. doi: 10.1016/j.ajhg.2024.05.016. Epub 2024 Jun 11. Am J Hum Genet. 2024. PMID: 38866022 Free PMC article.
-
Exploring the origins of neurodevelopmental proteasomopathies associated with cardiac malformations: are neural crest cells central to certain pathological mechanisms?Front Cell Dev Biol. 2024 Jul 12;12:1370905. doi: 10.3389/fcell.2024.1370905. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39071803 Free PMC article.
-
Immunogenic cell death triggered by impaired deubiquitination in multiple myeloma relies on dysregulated type I interferon signaling.Front Immunol. 2023 Mar 2;14:982720. doi: 10.3389/fimmu.2023.982720. eCollection 2023. Front Immunol. 2023. PMID: 36936919 Free PMC article.
-
Targeting the hallmarks of aging to improve influenza vaccine responses in older adults.Immun Ageing. 2023 May 17;20(1):23. doi: 10.1186/s12979-023-00348-6. Immun Ageing. 2023. PMID: 37198683 Free PMC article. Review.
-
Chronic inflammation and the hallmarks of aging.Mol Metab. 2023 Aug;74:101755. doi: 10.1016/j.molmet.2023.101755. Epub 2023 Jun 15. Mol Metab. 2023. PMID: 37329949 Free PMC article. Review.
References
-
- Plytycz B., Seljelid R. From inflammation to sickness: Historical perspective. Arch. Immunol. Exp. (Warsz) 2003;51:105–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

