DOT1L Methyltransferase Regulates Calcium Influx in Erythroid Progenitor Cells in Response to Erythropoietin
- PMID: 35563527
- PMCID: PMC9099724
- DOI: 10.3390/ijms23095137
DOT1L Methyltransferase Regulates Calcium Influx in Erythroid Progenitor Cells in Response to Erythropoietin
Abstract
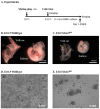
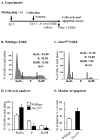
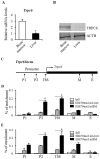
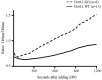
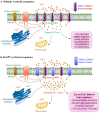
Erythropoietin (EPO) signaling plays a vital role in erythropoiesis by regulating proliferation and lineage-specific differentiation of murine hematopoietic progenitor cells (HPCs). An important downstream response of EPO signaling is calcium (Ca2+) influx, which is regulated by transient receptor potential channel (TRPC) proteins, particularly TRPC2 and TRPC6. While EPO induces Ca2+ influx through TRPC2, TRPC6 inhibits the function of TRPC2. Thus, interactions between TRPC2 and TRPC6 regulate the rate of Ca2+ influx in EPO-induced erythropoiesis. In this study, we observed that the expression of TRPC6 in KIT-positive erythroid progenitor cells was regulated by DOT1L. DOT1L is a methyltransferase that plays an important role in many biological processes during embryonic development including early erythropoiesis. We previously reported that Dot1l knockout (Dot1lKO) HPCs in the yolk sac failed to develop properly, which resulted in lethal anemia. In this study, we detected a marked downregulation of Trpc6 gene expression in Dot1lKO progenitor cells in the yolk sac compared to the wild type (WT). The promoter and the proximal regions of the Trpc6 gene locus exhibited an enrichment of H3K79 methylation, which is mediated solely by DOT1L. However, the expression of Trpc2, the positive regulator of Ca2+ influx, remained unchanged, resulting in an increased TRPC2/TRPC6 ratio. As the loss of DOT1L decreased TRPC6, which inhibited Ca2+ influx by TRPC2, Dot1lKO HPCs in the yolk sac exhibited accelerated and sustained elevated levels of Ca2+ influx. Such heightened Ca2+ levels might have detrimental effects on the growth and proliferation of HPCs in response to EPO.
Keywords: DOT1L; TRPC6; calcium influx; erythroid progenitors; erythropoietin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

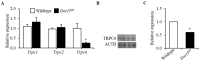
Similar articles
-
Interaction of TRPC2 and TRPC6 in erythropoietin modulation of calcium influx.J Biol Chem. 2004 Mar 12;279(11):10514-22. doi: 10.1074/jbc.M308478200. Epub 2003 Dec 29. J Biol Chem. 2004. PMID: 14699131
-
TRPC3 activation by erythropoietin is modulated by TRPC6.J Biol Chem. 2009 Feb 13;284(7):4567-81. doi: 10.1074/jbc.M804734200. Epub 2008 Dec 13. J Biol Chem. 2009. PMID: 19074769 Free PMC article.
-
Erythropoietin modulates calcium influx through TRPC2.J Biol Chem. 2002 Sep 13;277(37):34375-82. doi: 10.1074/jbc.M205541200. Epub 2002 Jul 11. J Biol Chem. 2002. PMID: 12167663
-
The role of tyrosine phosphorylation in proliferation and maturation of erythroid progenitor cells--signals emanating from the erythropoietin receptor.Eur J Biochem. 1997 Nov 1;249(3):637-47. doi: 10.1111/j.1432-1033.1997.t01-1-00637.x. Eur J Biochem. 1997. PMID: 9395308 Review.
-
Role of c-Kit and erythropoietin receptor in erythropoiesis.Crit Rev Oncol Hematol. 2005 Apr;54(1):63-75. doi: 10.1016/j.critrevonc.2004.11.005. Crit Rev Oncol Hematol. 2005. PMID: 15780908 Review.
Cited by
-
Activating mutations in JAK2 and CALR differentially affect intracellular calcium flux in store operated calcium entry.Cell Commun Signal. 2024 Mar 21;22(1):186. doi: 10.1186/s12964-024-01530-z. Cell Commun Signal. 2024. PMID: 38509561 Free PMC article.
-
DOT1L Mediated Gene Repression in Extensively Self-Renewing Erythroblasts.Front Genet. 2022 Mar 23;13:828086. doi: 10.3389/fgene.2022.828086. eCollection 2022. Front Genet. 2022. PMID: 35401699 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

