SARS-CoV-2 Infection Dysregulates Cilia and Basal Cell Homeostasis in the Respiratory Epithelium of Hamsters
- PMID: 35563514
- PMCID: PMC9102945
- DOI: 10.3390/ijms23095124
SARS-CoV-2 Infection Dysregulates Cilia and Basal Cell Homeostasis in the Respiratory Epithelium of Hamsters
Abstract
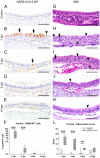
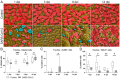
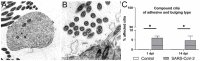
Similar to many other respiratory viruses, SARS-CoV-2 targets the ciliated cells of the respiratory epithelium and compromises mucociliary clearance, thereby facilitating spread to the lungs and paving the way for secondary infections. A detailed understanding of mechanism involved in ciliary loss and subsequent regeneration is crucial to assess the possible long-term consequences of COVID-19. The aim of this study was to characterize the sequence of histological and ultrastructural changes observed in the ciliated epithelium during and after SARS-CoV-2 infection in the golden Syrian hamster model. We show that acute infection induces a severe, transient loss of cilia, which is, at least in part, caused by cilia internalization. Internalized cilia colocalize with membrane invaginations, facilitating virus entry into the cell. Infection also results in a progressive decline in cells expressing the regulator of ciliogenesis FOXJ1, which persists beyond virus clearance and the termination of inflammatory changes. Ciliary loss triggers the mobilization of p73+ and CK14+ basal cells, which ceases after regeneration of the cilia. Although ciliation is restored after two weeks despite the lack of FOXJ1, an increased frequency of cilia with ultrastructural alterations indicative of secondary ciliary dyskinesia is observed. In summary, the work provides new insights into SARS-CoV-2 pathogenesis and expands our understanding of virally induced damage to defense mechanisms in the conducting airways.
Keywords: COVID-19; SARS-CoV-2; cilia; golden Syrian hamster; histology; immunohistochemistry; respiratory epithelium; scanning electron microscopy; trachea; transmission electron microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Live imaging of airway epithelium reveals that mucociliary clearance modulates SARS-CoV-2 spread.Nat Commun. 2024 Nov 2;15(1):9480. doi: 10.1038/s41467-024-53791-4. Nat Commun. 2024. PMID: 39488529 Free PMC article.
-
SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance.Nat Commun. 2021 Jul 16;12(1):4354. doi: 10.1038/s41467-021-24521-x. Nat Commun. 2021. PMID: 34272374 Free PMC article.
-
SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review.
-
Influenza A virus enhances ciliary activity and mucociliary clearance via TLR3 in airway epithelium.Respir Res. 2020 Oct 27;21(1):282. doi: 10.1186/s12931-020-01555-1. Respir Res. 2020. PMID: 33109186 Free PMC article.
-
Hamsters as a Model of Severe Acute Respiratory Syndrome Coronavirus-2.Comp Med. 2021 Oct 1;71(5):398-410. doi: 10.30802/AALAS-CM-21-000036. Epub 2021 Sep 29. Comp Med. 2021. PMID: 34588095 Free PMC article. Review.
Cited by
-
Chronic lung inflammation and CK14+ basal cell proliferation induce persistent alveolar-bronchiolization in SARS-CoV-2-infected hamsters.EBioMedicine. 2024 Oct;108:105363. doi: 10.1016/j.ebiom.2024.105363. Epub 2024 Sep 25. EBioMedicine. 2024. PMID: 39326207 Free PMC article.
-
The Interplay between Airway Cilia and Coronavirus Infection, Implications for Prevention and Control of Airway Viral Infections.Cells. 2024 Aug 14;13(16):1353. doi: 10.3390/cells13161353. Cells. 2024. PMID: 39195243 Free PMC article. Review.
-
Live imaging of airway epithelium reveals that mucociliary clearance modulates SARS-CoV-2 spread.Nat Commun. 2024 Nov 2;15(1):9480. doi: 10.1038/s41467-024-53791-4. Nat Commun. 2024. PMID: 39488529 Free PMC article.
-
In vivo inhibition of nuclear ACE2 translocation protects against SARS-CoV-2 replication and lung damage through epigenetic imprinting.Nat Commun. 2023 Jun 27;14(1):3680. doi: 10.1038/s41467-023-39341-4. Nat Commun. 2023. PMID: 37369668 Free PMC article.
-
The alphaherpesvirus gE/gI glycoprotein complex and proteases jointly orchestrate invasion across the host's upper respiratory epithelial barrier.mBio. 2024 Nov 13;15(11):e0187324. doi: 10.1128/mbio.01873-24. Epub 2024 Oct 9. mBio. 2024. PMID: 39382295 Free PMC article.
References
-
- Ravindra N.G., Alfajaro M.M., Gasque V., Huston N.C., Wan H., Szigeti-Buck K., Yasumoto Y., Greaney A.M., Habet V., Chow R.D., et al. Single-cell longitudinal analysis of SARS-CoV-2 infection in human airway epithelium identifies target cells, alterations in gene expression, and cell state changes. PLoS Biol. 2021;19:e3001143. doi: 10.1371/journal.pbio.3001143. - DOI - PMC - PubMed
-
- Tomashefski J.F., Farver C.F. Anatomy and Histology of the Lung. In: Tomashefski J.F., Cagle P.T., Farver C.F., Fraire A.E., editors. Dail and Hammar’s Pulmonary Pathology: Volume I: Nonneoplastic Lung Disease. Springer; New York, NY, USA: 2008. pp. 20–48.
MeSH terms
Grants and funding
- LE 824/10-1/Deutsche Forschungsgemeinschaft
- Open Access Publication Costs/University of Veterinary Medicine Hannover, Foundation
- 398066876/GRK 2485/1/Deutsche Forschungsgemeinschaft
- RAPID (Risk assessment in re-pandemic respiratory infectious diseases), 01KI1723G/Federal Ministry of Education and Research
- 14-76103-184 CORONA-15/20/Niedersächsische Ministerium für Wissenschaft und Kultur
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

