Effects of Low-Intensity and Long-Term Aerobic Exercise on the Psoas Muscle of mdx Mice: An Experimental Model of Duchenne Muscular Dystrophy
- PMID: 35562874
- PMCID: PMC9105402
- DOI: 10.3390/ijms23094483
Effects of Low-Intensity and Long-Term Aerobic Exercise on the Psoas Muscle of mdx Mice: An Experimental Model of Duchenne Muscular Dystrophy
Abstract
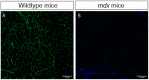
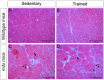
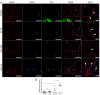
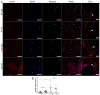
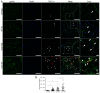
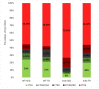
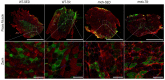
Duchenne muscular dystrophy (DMD) is a muscle disease characterized by the absence of the protein dystrophin, which causes a loss of sarcolemma integrity, determining recurrent muscle injuries, decrease in muscle function, and progressive degeneration. Currently, there is a need for therapeutic treatments to improve the quality of life of DMD patients. Here, we investigated the effects of a low-intensity aerobic training (37 sessions) on satellite cells, peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α protein (PGC-1α), and different types of fibers of the psoas muscle from mdx mice (DMD experimental model). Wildtype and mdx mice were randomly divided into sedentary and trained groups (n = 24). Trained animals were subjected to 37 sessions of low-intensity running on a motorized treadmill. Subsequently, the psoas muscle was excised and analyzed by immunofluorescence for dystrophin, satellite cells, myosin heavy chain (MHC), and PGC-1α content. The minimal Feret's diameters of the fibers were measured, and light microscopy was applied to observe general morphological features of the muscles. The training (37 sessions) improved morphological features in muscles from mdx mice and caused an increase in the number of quiescent/activated satellite cells. It also increased the content of PGC-1α in the mdx group. We concluded that low-intensity aerobic exercise (37 sessions) was able to reverse deleterious changes determined by DMD.
Keywords: Duchenne muscular dystrophy; PGC-1α; immunofluorescence; low-intensity aerobic exercise; mdx mice; satellite cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Long-Term Protective Effect of Human Dystrophin Expressing Chimeric (DEC) Cell Therapy on Amelioration of Function of Cardiac, Respiratory and Skeletal Muscles in Duchenne Muscular Dystrophy.Stem Cell Rev Rep. 2022 Dec;18(8):2872-2892. doi: 10.1007/s12015-022-10384-2. Epub 2022 May 19. Stem Cell Rev Rep. 2022. PMID: 35590083 Free PMC article.
-
Isometric resistance training increases strength and alters histopathology of dystrophin-deficient mouse skeletal muscle.J Appl Physiol (1985). 2019 Feb 1;126(2):363-375. doi: 10.1152/japplphysiol.00948.2018. Epub 2018 Dec 20. J Appl Physiol (1985). 2019. PMID: 30571283 Free PMC article.
-
Rescue of dystrophic skeletal muscle by PGC-1α involves restored expression of dystrophin-associated protein complex components and satellite cell signaling.Am J Physiol Regul Integr Comp Physiol. 2013 Jul 1;305(1):R13-23. doi: 10.1152/ajpregu.00221.2012. Epub 2013 Apr 17. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23594613
-
Brain function in Duchenne muscular dystrophy.Brain. 2002 Jan;125(Pt 1):4-13. doi: 10.1093/brain/awf012. Brain. 2002. PMID: 11834588 Review.
-
Bone tissue and muscle dystrophin deficiency in mdx mice.Joint Bone Spine. 2012 Mar;79(2):129-33. doi: 10.1016/j.jbspin.2011.08.004. Epub 2011 Nov 12. Joint Bone Spine. 2012. PMID: 22079415 Review.
Cited by
-
Roles of super enhancers and enhancer RNAs in skeletal muscle development and disease.Cell Cycle. 2023 Mar;22(5):495-505. doi: 10.1080/15384101.2022.2129240. Epub 2022 Oct 2. Cell Cycle. 2023. PMID: 36184878 Free PMC article. Review.
References
-
- Santos N.M., Rezende M.D.M., Terni A., Hayashi M.C.B., Fávero F.M., Quadros A.A.J., Reis L.I.O., dos Adissi M., Langer A.L., Fontes S.V., et al. Perfil clínico e funcional dos pacientes com Distrofia Muscular de Duchenne assistidos na Associação Brasileira de Distrofia Muscular (ABDIM) attending the Brazilian Association of muscular dystrophy (ABDIM) Rev. Neurocienc. 2006;14:015–022. doi: 10.34024/rnc.2006.v14.8782. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

