Safety and efficacy of pegylated recombinant human granulocyte colony-stimulating factor during concurrent chemoradiotherapy for small-cell lung cancer: a retrospective, cohort-controlled trial
- PMID: 35562713
- PMCID: PMC9107159
- DOI: 10.1186/s12885-022-09644-8
Safety and efficacy of pegylated recombinant human granulocyte colony-stimulating factor during concurrent chemoradiotherapy for small-cell lung cancer: a retrospective, cohort-controlled trial
Abstract
Objective: To investigate pegylated recombinant human granulocyte colony-stimulating factor (PEG-rhG-CSF) safety and efficacy in preventing hematological toxicity during concurrent chemoradiotherapy (CCRT) for small-cell lung cancer (SCLC).
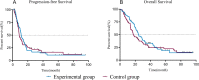
Methods: We retrospectively assessed 80 SCLC patients treated with CCRT from January 2013 to December 2018 who received PEG-rhG-CSF within 48 hours after the end of chemotherapy, defined as prophylactic use, as the experimental group. An additional 80 patients who were not treated with PEG-rhG-CSF were matched 1:1 by the propensity score matching method and served as the control group. The main observations were differences in hematological toxicity, neutrophil changes, febrile neutropenia (FN) incidence and adverse reactions. Progression-free survival (PFS) and overall survival (OS) were analyzed with regular assessment and follow-up.
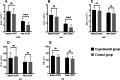
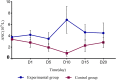
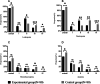
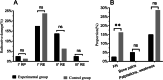
Results: The leukocyte, neutrophil, erythrocyte, and platelet counts and hemoglobin level decreased after CCRT, but the experimental group had slightly higher leukocyte and neutrophil counts than the control group (P < 0.05). The incidences of grade III-IV leukopenia (18.75% vs. 61.25%) and neutropenia (23.75% vs. 67.5%) in the experimental group were significantly lower than those in the control group (P < 0.05). The absolute neutrophil count was 4.17 ± 0.79 (× 109/L) on day 1 and peaked 6.81 ± 2.37 (× 109/L) on day 10 in the experimental group; the value in the control group was 2.81 ± 0.86 (× 109/L) on day 1. It decreased significantly and reached the minimum 0.91 ± 0.53 (× 109/L) on day 10 (P < 0.05). The experimental group had a lower FN incidence than the control group (P < 0.05). There was also no significant acute esophagitis or pulmonary toxicity. The treatment had no significant effect on PFS (11.4 months vs. 8.7 months, P = 0.958) or OS (23.9 months vs. 17.3 months, P = 0.325) over an 18.6-month median follow-up time.
Conclusion: PEG-rhG-CSF has good efficacy and safety in preventing hematological toxicity in SCLC patients during CCRT and has no significant effects on PFS or OS.
Keywords: Febrile neutropenia; Hematological toxicity; PEG-rhG-CSF; Radiotherapy; Survival time.
© 2022. The Author(s).
Conflict of interest statement
All authors have no conflicts of interest to declare.
Figures
Similar articles
-
Efficacy and safety of 3 mg pegylated recombinant human granulocyte colony-stimulating factor as support to chemotherapy for lung cancer.Thorac Cancer. 2022 Jan;13(1):117-125. doi: 10.1111/1759-7714.14233. Epub 2021 Nov 17. Thorac Cancer. 2022. PMID: 34791805 Free PMC article. Clinical Trial.
-
A clinical study of pegylated recombinant human granulocyte colony stimulating factor (PEG-rhG-CSF) in preventing neutropenia during concurrent chemoradiotherapy of cervical cancer.BMC Cancer. 2021 Jun 2;21(1):661. doi: 10.1186/s12885-021-08364-9. BMC Cancer. 2021. PMID: 34078317 Free PMC article. Clinical Trial.
-
Pegylated recombinant human granulocyte colony-stimulating factor regulates the immune status of patients with small cell lung cancer.Thorac Cancer. 2020 Mar;11(3):713-722. doi: 10.1111/1759-7714.13322. Epub 2020 Feb 5. Thorac Cancer. 2020. PMID: 32020764 Free PMC article.
-
A retrospective evaluation of therapeutic efficacy and safety of chemoradiotherapy in older patients (aged ≥ 75 years) with limited-disease small cell lung cancer: insights from two institutions and review of the literature.Radiol Oncol. 2024 Sep 15;58(3):432-443. doi: 10.2478/raon-2024-0054. eCollection 2024 Sep 1. Radiol Oncol. 2024. PMID: 39287161 Free PMC article. Review.
-
Prophylactic antibiotics or G(M)-CSF for the prevention of infections and improvement of survival in cancer patients receiving myelotoxic chemotherapy.Cochrane Database Syst Rev. 2015 Dec 21;2015(12):CD007107. doi: 10.1002/14651858.CD007107.pub3. Cochrane Database Syst Rev. 2015. PMID: 26687844 Free PMC article. Review.
Cited by
-
Clinical and genetic characteristics of patients with TRG 0 and TRG III in esophageal squamous cell carcinoma after neoadjuvant therapy.Sci Rep. 2024 Jul 31;14(1):17708. doi: 10.1038/s41598-024-68820-x. Sci Rep. 2024. PMID: 39085429 Free PMC article.
-
Therapeutic efficacy of rare earth carbonate with chemoradiotherapy in late-stage non-small cell lung cancer: a cohort prospective study.Front Endocrinol (Lausanne). 2023 Dec 15;14:1301032. doi: 10.3389/fendo.2023.1301032. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38192415 Free PMC article.
-
A Prognostic Model Based on Nutritional Indexes for Patients With Pan-Cancer: A Real-World Cohort Study.Cancer Rep (Hoboken). 2024 Jun;7(6):e2121. doi: 10.1002/cnr2.2121. Cancer Rep (Hoboken). 2024. PMID: 39031861 Free PMC article.
References
-
- Zhang X. Interpretation of CSCO guidelines for the diagnosis and treatment of small cell lung Cancer in 2020. J Clin Int Med. 2020;37(11):820–822. doi: 10.3969/j.issn.1001-9057.2020.11.021. - DOI
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859. - DOI - PubMed
-
- Chen M, Wang LH. Clinical guidelines for radiotherapy of small cell lung Cancer in China (2020 edition) Chin J Radiat Oncol. 2020;29(08):608–614. doi: 10.3760/cma.j.cn113030-20200528-00285. - DOI
-
- Bunn PA, Crowley J, Kelly K, Hazuka MB, Beasley K, Upchurch C, Livingston R, Weiss GR, Hicks WJ, Gandara DR. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the southwest oncology group. J Clin Oncol. 1995;13(7):1632–1641. doi: 10.1200/JCO.1995.13.7.1632. - DOI - PubMed
-
- C.M.A. Chinese Society of Hematology, H.B Chinese Medical Doctor Association, [Chinese guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever (2020)] Zhonghua Xue Ye Xue Za Zhi. 2020;41(12):969–978. doi: 10.3760/cma.j.issn.0253-2727.2020.12.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

