Improving Association Studies and Genomic Predictions for Climbing Beans With Data From Bush Bean Populations
- PMID: 35557726
- PMCID: PMC9085748
- DOI: 10.3389/fpls.2022.830896
Improving Association Studies and Genomic Predictions for Climbing Beans With Data From Bush Bean Populations
Abstract
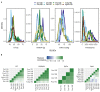
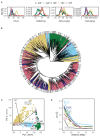
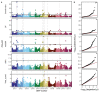
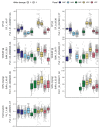
Common bean (Phaseolus vulgaris L.) has two major origins of domestication, Andean and Mesoamerican, which contribute to the high diversity of growth type, pod and seed characteristics. The climbing growth habit is associated with increased days to flowering (DF), seed iron concentration (SdFe), nitrogen fixation, and yield. However, breeding efforts in climbing beans have been limited and independent from bush type beans. To advance climbing bean breeding, we carried out genome-wide association studies and genomic predictions using 1,869 common bean lines belonging to five breeding panels representing both gene pools and all growth types. The phenotypic data were collected from 17 field trials and were complemented with 16 previously published trials. Overall, 38 significant marker-trait associations were identified for growth habit, 14 for DF, 13 for 100 seed weight, three for SdFe, and one for yield. Except for DF, the results suggest a common genetic basis for traits across all panels and growth types. Seven QTL associated with growth habits were confirmed from earlier studies and four plausible candidate genes for SdFe and 100 seed weight were newly identified. Furthermore, the genomic prediction accuracy for SdFe and yield in climbing beans improved up to 8.8% when bush-type bean lines were included in the training population. In conclusion, a large population from different gene pools and growth types across multiple breeding panels increased the power of genomic analyses and provides a solid and diverse germplasm base for genetic improvement of common bean.
Keywords: climbing and bush type bean; common bean (Phaseolus vulgaris L.); genome-wide association studies (GWAS); genomic selection; growth habit; pleiotropy; population structure.
Copyright © 2022 Keller, Ariza-Suarez, Portilla-Benavides, Buendia, Aparicio, Amongi, Mbiu, Msolla, Miklas, Porch, Burridge, Mukankusi, Studer and Raatz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Novel candidate loci for morpho-agronomic and seed quality traits detected by targeted genotyping-by-sequencing in common bean.Front Plant Sci. 2022 Nov 10;13:1014282. doi: 10.3389/fpls.2022.1014282. eCollection 2022. Front Plant Sci. 2022. PMID: 36438107 Free PMC article.
-
Genetic mapping for agronomic traits in a MAGIC population of common bean (Phaseolus vulgaris L.) under drought conditions.BMC Genomics. 2020 Nov 16;21(1):799. doi: 10.1186/s12864-020-07213-6. BMC Genomics. 2020. PMID: 33198642 Free PMC article.
-
Genome-Wide Association Studies Detect Multiple QTLs for Productivity in Mesoamerican Diversity Panel of Common Bean Under Drought Stress.Front Plant Sci. 2020 Nov 12;11:574674. doi: 10.3389/fpls.2020.574674. eCollection 2020. Front Plant Sci. 2020. PMID: 33343591 Free PMC article.
-
A Review of Angular Leaf Spot Resistance in Common Bean.Crop Sci. 2019 Jul-Aug;59(4):1376-1391. doi: 10.2135/cropsci2018.09.0596. Epub 2019 Jun 4. Crop Sci. 2019. PMID: 33343018 Free PMC article. Review.
-
Factors Influencing the Emergence of Heterogeneous Populations of Common Bean (Phaseolus vulgaris L.) and Their Potential for Intercropping.Plants (Basel). 2024 Apr 16;13(8):1112. doi: 10.3390/plants13081112. Plants (Basel). 2024. PMID: 38674521 Free PMC article. Review.
Cited by
-
Novel candidate loci for morpho-agronomic and seed quality traits detected by targeted genotyping-by-sequencing in common bean.Front Plant Sci. 2022 Nov 10;13:1014282. doi: 10.3389/fpls.2022.1014282. eCollection 2022. Front Plant Sci. 2022. PMID: 36438107 Free PMC article.
-
Genome-wide association mapping for component traits of drought tolerance in dry beans (Phaseolus vulgaris L.).PLoS One. 2023 May 18;18(5):e0278500. doi: 10.1371/journal.pone.0278500. eCollection 2023. PLoS One. 2023. PMID: 37200295 Free PMC article.
-
Linking photosynthesis and yield reveals a strategy to improve light use efficiency in a climbing bean breeding population.J Exp Bot. 2024 Feb 2;75(3):901-916. doi: 10.1093/jxb/erad416. J Exp Bot. 2024. PMID: 37878015 Free PMC article.
-
GWAS of resistance to three bacterial diseases in the Andean common bean diversity panel.Front Plant Sci. 2024 Sep 5;15:1469381. doi: 10.3389/fpls.2024.1469381. eCollection 2024. Front Plant Sci. 2024. PMID: 39301162 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

