Insights from C. elegans into Microsporidia Biology and Host-Pathogen Relationships
- PMID: 35544001
- PMCID: PMC9208714
- DOI: 10.1007/978-3-030-93306-7_5
Insights from C. elegans into Microsporidia Biology and Host-Pathogen Relationships
Abstract
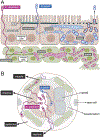
Microsporidia are poorly understood, ubiquitous eukaryotic parasites that are completely dependent on their hosts for replication. With the discovery of microsporidia species naturally infecting the genetically tractable transparent nematode C. elegans, this host has been used to explore multiple areas of microsporidia biology. Here we review results about microsporidia infections in C. elegans, which began with the discovery of the intestinal-infecting species Nematocida parisii. Recent findings include new species identification in the Nematocida genus, with more intestinal-infecting species, and also a species with broader tissue tropism, the epidermal and muscle-infecting species Nematocida displodere. This species has a longer polar tube infection apparatus, which may enable its wider tissue range. After invasion, multiple Nematocida species appear to fuse host cells, which likely promotes their dissemination within host organs. Localized proteomics identified Nematocida proteins that have direct contact with the C. elegans intestinal cytosol and nucleus, and many of these host-exposed proteins belong to expanded, species-specific gene families. On the host side, forward genetic screens have identified regulators of the Intracellular Pathogen Response (IPR), which is a transcriptional response induced by both microsporidia and the Orsay virus, which is also a natural, obligate intracellular pathogen of the C. elegans intestine. The IPR constitutes a novel immune/stress response that promotes resistance against microsporidia, virus, and heat shock. Overall, the Nematocida/C. elegans system has provided insights about strategies for microsporidia pathogenesis, as well as innate defense pathways against these parasites.
Keywords: C. elegans; Host-exposed proteins; Intracellular Pathogen Response; Microsporidia; Nematocida; Syncytium; Tissue tropism.
© 2022. The Author(s), under exclusive license to Springer Nature Switzerland AG.
Conflict of interest statement
Figures


Similar articles
-
A Large Collection of Novel Nematode-Infecting Microsporidia and Their Diverse Interactions with Caenorhabditis elegans and Other Related Nematodes.PLoS Pathog. 2016 Dec 12;12(12):e1006093. doi: 10.1371/journal.ppat.1006093. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27942022 Free PMC article.
-
An intestinally secreted host factor promotes microsporidia invasion of C. elegans.Elife. 2022 Jan 7;11:e72458. doi: 10.7554/eLife.72458. Elife. 2022. PMID: 34994689 Free PMC article.
-
Microsporidia Intracellular Development Relies on Myc Interaction Network Transcription Factors in the Host.G3 (Bethesda). 2016 Sep 8;6(9):2707-16. doi: 10.1534/g3.116.029983. G3 (Bethesda). 2016. PMID: 27402359 Free PMC article.
-
Host-Microsporidia Interactions in Caenorhabditis elegans, a Model Nematode Host.Microbiol Spectr. 2016 Oct;4(5). doi: 10.1128/microbiolspec.FUNK-0003-2016. Microbiol Spectr. 2016. PMID: 27763260 Review.
-
Microsporidia: Pervasive natural pathogens of Caenorhabditis elegans and related nematodes.J Eukaryot Microbiol. 2024 Sep-Oct;71(5):e13027. doi: 10.1111/jeu.13027. Epub 2024 May 3. J Eukaryot Microbiol. 2024. PMID: 38702921 Review.
Cited by
-
Genomic and phenotypic evolution of nematode-infecting microsporidia.PLoS Pathog. 2023 Jul 20;19(7):e1011510. doi: 10.1371/journal.ppat.1011510. eCollection 2023 Jul. PLoS Pathog. 2023. PMID: 37471459 Free PMC article.
-
Multiple pals gene modules control a balance between immunity and development in Caenorhabditis elegans.bioRxiv [Preprint]. 2023 Jan 18:2023.01.15.524171. doi: 10.1101/2023.01.15.524171. bioRxiv. 2023. Update in: PLoS Pathog. 2023 Jul 18;19(7):e1011120. doi: 10.1371/journal.ppat.1011120 PMID: 36711775 Free PMC article. Updated. Preprint.
-
A pals-25 gain-of-function allele triggers systemic resistance against natural pathogens of C. elegans.PLoS Genet. 2022 Oct 3;18(10):e1010314. doi: 10.1371/journal.pgen.1010314. eCollection 2022 Oct. PLoS Genet. 2022. PMID: 36191002 Free PMC article.
-
Caenorhabditis elegans RIG-I-like receptor DRH-1 signals via CARDs to activate antiviral immunity in intestinal cells.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2402126121. doi: 10.1073/pnas.2402126121. Epub 2024 Jul 9. Proc Natl Acad Sci U S A. 2024. PMID: 38980902 Free PMC article.
-
Conservation of Nematocida microsporidia gene expression and host response in Caenorhabditis nematodes.PLoS One. 2022 Dec 19;17(12):e0279103. doi: 10.1371/journal.pone.0279103. eCollection 2022. PLoS One. 2022. PMID: 36534656 Free PMC article.
