Mild polyaddition and polyalkylation based on the carbon-carbon bond formation reaction of active methylene
- PMID: 35542661
- PMCID: PMC9076256
- DOI: 10.1039/c9ra08155k
Mild polyaddition and polyalkylation based on the carbon-carbon bond formation reaction of active methylene
Abstract
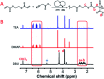
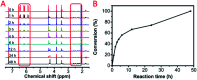
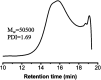
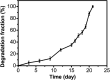
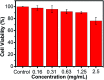
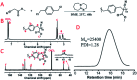
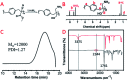
The Michael addition and alkylation reaction of active methylene compounds (AMCs) with two active hydrogens had been investigated extensively in organic chemistry, while the polymerization of AMCs had few studies. Herein, we reported active methylene-based polyaddition and polyalkylation catalyzed via an organic superbase under ambient conditions. A model polymerization was first conducted between ethylene glycol diacrylate (EGDA) and methyl cyanoacetate (MCA). The molecular weight (M w) of the model polymer was up to 50 500 g mol-1 with a high yield (99%). Eight AMCs were selected and a high-throughput parallel synthesizing instrument (HTPSI) was used to synthesize semi-library polymers of AMCs and EGDA via a Michael type polyaddition. The obtained AMC-based polymers had low cell cytotoxicity. Elastomers with cyanogen groups could be prepared using trimethylolpropane triacrylate (TMPTA) as a crosslinker. Furthermore, three dihalogen compounds were explored to polymerize with MCA and malononitrile via alkylation reactions. The pendent cyanogen or ester groups of the polymers could be reduced by lithium aluminum hydride. Novel polymer families were constructed based on the polyaddition and polyalkylation of AMCs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Poly(β-trimethylsilyloxy ester): A Degradable Polymer Based on Retro Mukaiyama Aldol Reaction.Macromol Rapid Commun. 2022 Mar;43(6):e2100808. doi: 10.1002/marc.202100808. Epub 2022 Feb 19. Macromol Rapid Commun. 2022. PMID: 35142413
-
Chemoselective Lewis pair polymerization of renewable multivinyl-functionalized γ-butyrolactones.Philos Trans A Math Phys Eng Sci. 2017 Aug 28;375(2101):20170003. doi: 10.1098/rsta.2017.0003. Philos Trans A Math Phys Eng Sci. 2017. PMID: 28739962 Free PMC article.
-
Polyphosphoester-Camptothecin Prodrug with Reduction-Response Prepared via Michael Addition Polymerization and Click Reaction.ACS Appl Mater Interfaces. 2017 Apr 26;9(16):13939-13949. doi: 10.1021/acsami.7b02281. Epub 2017 Apr 11. ACS Appl Mater Interfaces. 2017. PMID: 28378998
-
Recent results on functional polymers and macromonomers of interest as biomaterials or for biomaterial modification.Biomaterials. 1994 Dec;15(15):1235-41. doi: 10.1016/0142-9612(94)90275-5. Biomaterials. 1994. PMID: 7703320 Review.
-
Functional Polymers and Polymeric Materials From Renewable Alpha-Unsaturated Gamma-Butyrolactones.Front Chem. 2019 Dec 13;7:845. doi: 10.3389/fchem.2019.00845. eCollection 2019. Front Chem. 2019. PMID: 31921769 Free PMC article. Review.
References
-
- Qin A. J. Jim C. K. W. Lu W. X. Lam J. W. Y. Häussler M. Dong Y. Q. Sung H. H. Y. Williams I. D. Wong G. K. L. Tang B. Z. Macromolecules. 2007;40:2308–2317. doi: 10.1021/ma062859s. - DOI
-
- Shipp D. A. Polym. Rev. 2011;51:99–103. doi: 10.1080/15583724.2011.566406. - DOI
-
- Konkolewicz D. Wang Y. Zhong M. J. Krys P. Isse A. A. Gennaro A. Matyjaszewski K. Macromolecules. 2013;46:8749–8772. doi: 10.1021/ma401243k. - DOI
-
- Wang Y. Zhong M. J. Zhu W. P. Peng C. H. Zhang Y. Z. Konkolewicz D. Bortolamei N. Isse A. A. Gennaro A. Matyjaszewski K. Macromolecules. 2013;46:3793–3802. doi: 10.1021/ma400149t. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

