STUB1 regulates antiviral RNAi through inducing ubiquitination and degradation of Dicer and AGO2 in mammals
- PMID: 35533808
- PMCID: PMC9437610
- DOI: 10.1016/j.virs.2022.05.001
STUB1 regulates antiviral RNAi through inducing ubiquitination and degradation of Dicer and AGO2 in mammals
Abstract
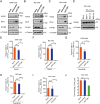
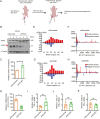
RNA interference (RNAi) is an intrinsic antiviral immune mechanism conserved in diverse eukaryotic organisms. However, the mechanism by which antiviral RNAi in mammals is regulated is poorly understood. In this study, we uncovered that the E3 ubiquitin ligase STIP1 homology and U-box-containing protein 1 (STUB1) was a new regulator of the RNAi machinery in mammals. We found that STUB1 interacted with and ubiquitinated AGO2, and targeted it for degradation in a chaperon-dependent manner. STUB1 promoted the formation of Lys48 (K48)-linked polyubiquitin chains on AGO2, and facilitated AGO2 degradation through ubiquitin-proteasome system. In addition to AGO2, STUB1 also induced the protein degradation of AGO1, AGO3 and AGO4. Further investigation revealed that STUB1 also regulated Dicer's ubiquitination via K48-linked polyubiquitin and induced the degradation of Dicer as well as its specialized form, termed antiviral Dicer (aviDicer) that expresses in mammalian stem cells. Moreover, we found that STUB1 deficiency up-regulated Dicer and AGO2, thereby enhancing the RNAi response and efficiently inhibiting viral replication in mammalian cells. Using the newborn mouse model of Enterovirus A71 (EV-A71), we confirmed that STUB1 deficiency enhanced the virus-derived siRNAs production and antiviral RNAi, which elicited a potent antiviral effect against EV-A71 infection in vivo. In summary, our findings uncovered that the E3 ubiquitin ligase STUB1 was a general regulator of the RNAi machinery by targeting Dicer, aviDicer and AGO1-4. Moreover, STUB1 regulated the RNAi response through mediating the abundance of Dicer and AGO2 during viral infection, thereby providing novel insights into the regulation of antiviral RNAi in mammals.
Keywords: Antiviral RNAi; Argonaute 2 (AGO2); Dicer; STIP1 homology and U-box-containing protein 1 (STUB1).
Copyright © 2022 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
The E3 ubiquitin ligase STUB1 attenuates cell senescence by promoting the ubiquitination and degradation of the core circadian regulator BMAL1.J Biol Chem. 2020 Apr 3;295(14):4696-4708. doi: 10.1074/jbc.RA119.011280. Epub 2020 Feb 10. J Biol Chem. 2020. PMID: 32041778 Free PMC article.
-
Mechanism and Function of Antiviral RNA Interference in Mice.mBio. 2020 Aug 4;11(4):e03278-19. doi: 10.1128/mBio.03278-19. mBio. 2020. PMID: 32753500 Free PMC article.
-
Caenorhabditis elegans RIG-I Homolog Mediates Antiviral RNA Interference Downstream of Dicer-Dependent Biogenesis of Viral Small Interfering RNAs.mBio. 2017 Mar 21;8(2):e00264-17. doi: 10.1128/mBio.00264-17. mBio. 2017. PMID: 28325765 Free PMC article.
-
Dicer's role as an antiviral: still an enigma.Curr Opin Immunol. 2014 Feb;26:49-55. doi: 10.1016/j.coi.2013.10.015. Epub 2013 Nov 22. Curr Opin Immunol. 2014. PMID: 24556400 Free PMC article. Review.
-
Mammalian viral suppressors of RNA interference.Trends Biochem Sci. 2022 Nov;47(11):978-988. doi: 10.1016/j.tibs.2022.05.001. Epub 2022 May 23. Trends Biochem Sci. 2022. PMID: 35618579 Free PMC article. Review.
Cited by
-
Phosphorylation of AGO2 by TBK1 Promotes the Formation of Oncogenic miRISC in NSCLC.Adv Sci (Weinh). 2024 Apr;11(15):e2305541. doi: 10.1002/advs.202305541. Epub 2024 Feb 13. Adv Sci (Weinh). 2024. PMID: 38351659 Free PMC article.
-
Argonaute protein-based nucleic acid detection technology.Front Microbiol. 2023 Sep 8;14:1255716. doi: 10.3389/fmicb.2023.1255716. eCollection 2023. Front Microbiol. 2023. PMID: 37744931 Free PMC article. Review.
-
Role of protein Post-translational modifications in enterovirus infection.Front Microbiol. 2024 Feb 26;15:1341599. doi: 10.3389/fmicb.2024.1341599. eCollection 2024. Front Microbiol. 2024. PMID: 38596371 Free PMC article. Review.
-
CircDCBLD2 alleviates liver fibrosis by regulating ferroptosis via facilitating STUB1-mediated PARK7 ubiquitination degradation.J Gastroenterol. 2024 Mar;59(3):229-249. doi: 10.1007/s00535-023-02068-6. Epub 2024 Feb 3. J Gastroenterol. 2024. PMID: 38310161
References
-
- Bronevetsky Y., Villarino A.V., Eisley C.J., Barbeau R., Barczak A.J., Heinz G.A., Kremmer E., Heissmeyer V., McManus M.T., Erle D.J., Rao A., Ansel K.M. T cell activation induces proteasomal degradation of Argonaute and rapid remodeling of the microRNA repertoire. J. Exp. Med. 2013;210:417–432. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

