Native human collagen type I provides a viable physiologically relevant alternative to xenogeneic sources for tissue engineering applications: A comparative in vitro and in vivo study
- PMID: 35532208
- PMCID: PMC11103545
- DOI: 10.1002/jbm.b.35080
Native human collagen type I provides a viable physiologically relevant alternative to xenogeneic sources for tissue engineering applications: A comparative in vitro and in vivo study
Abstract
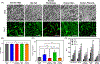
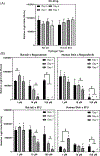
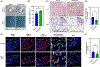
Xenogeneic sources of collagen type I remain a common choice for regenerative medicine applications due to ease of availability. Human and animal sources have some similarities, but small variations in amino acid composition can influence the physical properties of collagen, cellular response, and tissue remodeling. The goal of this work is to compare human collagen type I-based hydrogels versus animal-derived collagen type I-based hydrogels, generated from commercially available products, for their physico-chemical properties and for tissue engineering and regenerative medicine applications. Specifically, we evaluated whether the native human skin type I collagen could be used in the three most common research applications of this protein: as a substrate for attachment and proliferation of conventional 2D cell culture; as a source of matrix for a 3D cell culture; and as a source of matrix for tissue engineering. Results showed that species and tissue specific variations of collagen sources significantly impact the physical, chemical, and biological properties of collagen hydrogels including gelation kinetics, swelling ratio, collagen fiber morphology, compressive modulus, stability, and metabolic activity of hMSCs. Tumor constructs formulated with human skin collagen showed a differential response to chemotherapy agents compared to rat tail collagen. Human skin collagen performed comparably to rat tail collagen and enabled assembly of perfused human vessels in vivo. Despite differences in collagen manufacturing methods and supplied forms, the results suggest that commercially available human collagen can be used in lieu of xenogeneic sources to create functional scaffolds, but not all sources of human collagen behave similarly. These factors must be considered in the development of 3D tissues for drug screening and regenerative medicine applications.
Keywords: 3D construct; cancer modeling; collagen type I; hydrogel; xeno.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mohammad Z. Albanna is a founding member of Humabiologics, a start-up developing human-derived biomaterials for tissue engineering, which provided the HumaDerm products tested in this study. Mohammad Z. Albanna had no role in data collection. The other authors declare that they have no competing interests.
Figures


Similar articles
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
"It Is a Big Spider Web of Things": Sensory Experiences of Autistic Adults in Public Spaces.Autism Adulthood. 2023 Dec 1;5(4):411-422. doi: 10.1089/aut.2022.0024. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116051 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
Cited by
-
Species-Based Differences in Mechanical Properties, Cytocompatibility, and Printability of Methacrylated Collagen Hydrogels.Biomacromolecules. 2022 Dec 12;23(12):5137-5147. doi: 10.1021/acs.biomac.2c00985. Epub 2022 Nov 23. Biomacromolecules. 2022. PMID: 36417692 Free PMC article.
-
3D collagen microchamber arrays for combined chemotherapy effect evaluation on cancer cell numbers and migration.Biomicrofluidics. 2023 Jan 3;17(1):014101. doi: 10.1063/5.0121952. eCollection 2023 Jan. Biomicrofluidics. 2023. PMID: 36619874 Free PMC article.
References
-
- Silvipriya KS, Kumar KK, Bhat AR, et al. Collagen: animal sources and biomedical application. J Appl Pharm Sci. 2015;5:123–127.
-
- Lin YK, Liu DC. Comparison of physical–chemical properties of type I collagen from different species. Food Chem. 2006;99:244–251. doi:10.1016/j.foodchem.2005.06.053 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

