Shedding light on triple-negative breast cancer with Trop2-targeted antibody-drug conjugates
- PMID: 35530278
- PMCID: PMC9077081
Shedding light on triple-negative breast cancer with Trop2-targeted antibody-drug conjugates
Abstract
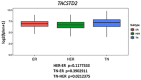
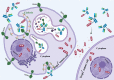
Triple-negative breast cancer (TNBC) is well-known as the most aggressive subtype of breast cancer. Because TNBC does not express Her2, estrogen receptor, and progesterone receptors, there had been no effective U.S. Food and Drug Administration-approved targeted therapy for it until PARP inhibitors and two PD-1/PD-L1 monoclonal antibodies were approved for treatment of TNBC. Most recently, an antibody-drug conjugate (ADC), called sacituzumab govitecan (SG), was approved for the treatment of TNBC patients previously received chemotherapy with advanced disease. SG consists of an anti-trophoblast cell-surface antigen 2 (Trop2) antibody conjugated with a topoisomerase I inhibitor, SN-38, which is diffused out of the targeted Trop2 positive cancer cells and induces the bystander killing effect on surrounding cells regardless of their Trop2 expression status. In the Phase III clinical trial, TNBC patients treated with SG showed significantly longer progression-free and overall survival compared to those who were received chemotherapy. In the present review, we summarized the cellular function and signaling of Trop2, the mechanism of action of SG, and the clinical trials of SG that led to its quick approval for TNBC. In addition, we introduced the current ongoing clinical trials of SG as well as another Trop2 ADC, which has potential to overcome some disadvantages of SG.
Keywords: SN-38; Triple-negative breast cancer; Trop2; antibody-drug conjugates; sacituzumab govitecan.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures


Similar articles
-
Sacituzumab govitecan: past, present and future of a new antibody-drug conjugate and future horizon.Future Oncol. 2022 Sep;18(28):3199-3215. doi: 10.2217/fon-2022-0407. Epub 2022 Sep 7. Future Oncol. 2022. PMID: 36069628 Review.
-
TROPION-Breast02: Datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative breast cancer.Future Oncol. 2023 Nov;19(35):2349-2359. doi: 10.2217/fon-2023-0228. Epub 2023 Aug 1. Future Oncol. 2023. PMID: 37526149
-
Trop2-targeted therapy in breast cancer.Biomark Res. 2024 Aug 13;12(1):82. doi: 10.1186/s40364-024-00633-6. Biomark Res. 2024. PMID: 39135109 Free PMC article. Review.
-
Breaking barriers in triple negative breast cancer (TNBC) - Unleashing the power of antibody-drug conjugates (ADCs).Cancer Treat Rev. 2024 Feb;123:102672. doi: 10.1016/j.ctrv.2023.102672. Epub 2023 Dec 14. Cancer Treat Rev. 2024. PMID: 38118302 Review.
-
Antibody-drug conjugates targeting TROP-2: Clinical development in metastatic breast cancer.Breast. 2022 Dec;66:169-177. doi: 10.1016/j.breast.2022.10.007. Epub 2022 Oct 18. Breast. 2022. PMID: 36302269 Free PMC article. Clinical Trial.
Cited by
-
Unraveling the Potential of ALK-Targeted Therapies in Non-Small Cell Lung Cancer: Comprehensive Insights and Future Directions.Biomedicines. 2024 Jan 27;12(2):297. doi: 10.3390/biomedicines12020297. Biomedicines. 2024. PMID: 38397899 Free PMC article. Review.
-
Sacituzumab govitecan for hormone receptor-positive and triple-negative breast cancers.Mol Cell Pharmacol. 2023;15(1):1-5. Mol Cell Pharmacol. 2023. PMID: 37090944 Free PMC article.
-
MRCK as a Potential Target for Claudin-Low Subtype of Breast Cancer.Int J Biol Sci. 2024 Jan 1;20(1):1-14. doi: 10.7150/ijbs.88285. eCollection 2024. Int J Biol Sci. 2024. PMID: 38164185 Free PMC article.
-
Comprehensive assessment of TECENTRIQ® and OPDIVO®: analyzing immunotherapy indications withdrawn in triple-negative breast cancer and hepatocellular carcinoma.Cancer Metastasis Rev. 2024 Sep;43(3):889-918. doi: 10.1007/s10555-024-10174-x. Epub 2024 Feb 27. Cancer Metastasis Rev. 2024. PMID: 38409546 Review.
-
CD24 is a novel target of chimeric antigen receptor T cells for the treatment of triple negative breast cancer.Cancer Immunol Immunother. 2023 Oct;72(10):3191-3202. doi: 10.1007/s00262-023-03491-7. Epub 2023 Jul 7. Cancer Immunol Immunother. 2023. PMID: 37418008 Free PMC article.
References
-
- Vankemmelbeke M, Durrant L. Third-generation antibody drug conjugates for cancer therapy--a balancing act. Ther Deliv. 2016;7:141–144. - PubMed
-
- Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, Cha JH, Xia W, Chan LC, Kim T, Chang SS, Lee HH, Chou CK, Liu YL, Yeh HC, Perillo EP, Dunn AK, Kuo CW, Khoo KH, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Huang TH, Sahin AA, Hortobagyi GN, Yoo SS, Hung MC. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33:187–201. e110. - PMC - PubMed
-
- Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, Cortes J, Soria JC, Curigliano G. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72:165–182. - PubMed
-
- Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin Cancer Res. 2006;12:6326–6330. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
