Silencing the odorant receptor co-receptor impairs olfactory reception in a sensillum-specific manner in the cockroach
- PMID: 35521537
- PMCID: PMC9065313
- DOI: 10.1016/j.isci.2022.104272
Silencing the odorant receptor co-receptor impairs olfactory reception in a sensillum-specific manner in the cockroach
Abstract
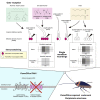
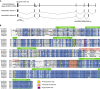
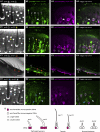
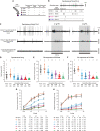
Insects detect odors via a large variety of odorant receptors (ORs) expressed in olfactory sensory neurons (OSNs). The insect OR is a heteromeric complex composed of a ligand-specific receptor and the co-receptor (ORco). In this study, we identified the ORco gene of the cockroach, Periplaneta americana (PameORco), and performed RNAi-based functional analysis of PameORco. All OSNs in the basiconic sensilla expressed PameORco and received a large variety of odors including sex pheromones. In trichoid sensilla, a PameORco-positive OSN was consistently paired with a PameORco-negative OSN tuned to acids. In adult cockroaches injected with PameORco dsRNA at the nymphal stage, the expression of PameORco, odor receptions via ORs, and its central processing were strongly suppressed. These results provide new insights into the molecular basis of olfactory reception in the cockroach. The long-lasting and irreversible effects of PameORco RNAi would be an effective method for controlling the household pest.
Keywords: Entomology; Molecular neuroscience; Sensory neuroscience.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Sensillum-specific, topographic projection patterns of olfactory receptor neurons in the antennal lobe of the cockroach Periplaneta americana.J Comp Neurol. 2012 Jun 1;520(8):1687-701. doi: 10.1002/cne.23007. J Comp Neurol. 2012. PMID: 22121009
-
Insect odorant receptor trafficking requires calmodulin.BMC Biol. 2016 Sep 29;14(1):83. doi: 10.1186/s12915-016-0306-x. BMC Biol. 2016. PMID: 27686128 Free PMC article.
-
The Sensilla-Specific Expression and Subcellular Localization of SNMP1 and SNMP2 Reveal Novel Insights into Their Roles in the Antenna of the Desert Locust Schistocerca gregaria.Insects. 2022 Jun 25;13(7):579. doi: 10.3390/insects13070579. Insects. 2022. PMID: 35886755 Free PMC article.
-
Odor Coding in Insects.In: Menini A, editor. The Neurobiology of Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. In: Menini A, editor. The Neurobiology of Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. PMID: 21882428 Free Books & Documents. Review.
-
The Two Main Olfactory Receptor Families in Drosophila, ORs and IRs: A Comparative Approach.Front Cell Neurosci. 2018 Aug 30;12:253. doi: 10.3389/fncel.2018.00253. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30214396 Free PMC article. Review.
Cited by
-
Cuticular hydrocarbon reception by sensory neurons in basiconic sensilla of the Japanese carpenter ant.Front Cell Neurosci. 2023 Feb 6;17:1084803. doi: 10.3389/fncel.2023.1084803. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36814868 Free PMC article.
-
Interactive parallel sex pheromone circuits that promote and suppress courtship behaviors in the cockroach.PNAS Nexus. 2024 Apr 15;3(4):pgae162. doi: 10.1093/pnasnexus/pgae162. eCollection 2024 Apr. PNAS Nexus. 2024. PMID: 38689705 Free PMC article.
-
Two sex pheromone receptors for sexual communication in the American cockroach.Sci China Life Sci. 2024 Jul;67(7):1455-1467. doi: 10.1007/s11427-023-2548-3. Epub 2024 Mar 22. Sci China Life Sci. 2024. PMID: 38523236
-
Genome-wide identification of candidate chemosensory receptors in the bean bug Riptortus pedestris (Hemiptera: Alydidae) and the functional verification of its odorant receptor co-receptor (Orco) in recognizing aggregation pheromone.Front Physiol. 2023 Jul 14;14:1224009. doi: 10.3389/fphys.2023.1224009. eCollection 2023. Front Physiol. 2023. PMID: 37520822 Free PMC article.
-
Stimulus duration encoding occurs early in the moth olfactory pathway.Commun Biol. 2024 Oct 3;7(1):1252. doi: 10.1038/s42003-024-06921-z. Commun Biol. 2024. PMID: 39363042 Free PMC article.
References
LinkOut - more resources
Full Text Sources

