Thermo-driven self-assembly of a PEG-containing amphiphile in a bilayer membrane
- PMID: 35518572
- PMCID: PMC9055338
- DOI: 10.1039/d0ra03920a
Thermo-driven self-assembly of a PEG-containing amphiphile in a bilayer membrane
Abstract
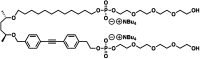
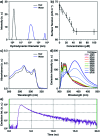
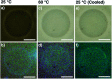
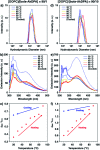
Self-assembly of lipid molecules in a plasma membrane, namely lipid raft formation, is involved in various dynamic functions of cells. Inspired by the raft formation observed in the cells, here we studied thermally induced self-assembly of a synthetic amphiphile, bola-AkDPA, in a bilayer membrane. The synthetic amphiphile consists of a hydrophobic unit including fluorescent aromatic and aliphatic components and hydrophilic tetraethylene glycol chains attached at both ends of the hydrophobic unit. In a polar solvent, bola-AkDPA formed aggregates to show excimer emission. In a lipid bilayer membrane, bola-AkDPA showed intensified excimer emission upon increase of its concentration or elevation of the temperature; bola-type amphiphiles containing oligoethylene glycol chains likely tend to form self-assemblies in a bilayer membrane triggered by thermal stimuli.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
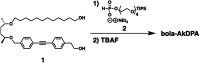
Similar articles
-
Contrasting Topological Effect of PEG-Containing Amphiphiles to Natural Lipids on Stability of Vesicles.Langmuir. 2016 May 10;32(18):4546-53. doi: 10.1021/acs.langmuir.6b00713. Epub 2016 Apr 27. Langmuir. 2016. PMID: 27093474
-
Thermally-induced lateral assembly of a PEG-containing amphiphile triggering vesicle budding.Chem Commun (Camb). 2017 Oct 24;53(85):11662-11665. doi: 10.1039/c7cc06489f. Chem Commun (Camb). 2017. PMID: 29018844
-
Amphiphilic building blocks for self-assembly: from amphiphiles to supra-amphiphiles.Acc Chem Res. 2012 Apr 17;45(4):608-18. doi: 10.1021/ar200226d. Epub 2012 Jan 13. Acc Chem Res. 2012. PMID: 22242811
-
Molecular Recognition Driven Bioinspired Directional Supramolecular Assembly of Amphiphilic (Macro)molecules and Proteins.Acc Chem Res. 2021 Jun 1;54(11):2670-2682. doi: 10.1021/acs.accounts.1c00195. Epub 2021 May 20. Acc Chem Res. 2021. PMID: 34014638 Review.
-
High-throughput development of amphiphile self-assembly materials: fast-tracking synthesis, characterization, formulation, application, and understanding.Acc Chem Res. 2013 Jul 16;46(7):1497-505. doi: 10.1021/ar300285u. Epub 2013 Feb 21. Acc Chem Res. 2013. PMID: 23427836 Review.
References
LinkOut - more resources
Full Text Sources

