FGF9 Alleviates the Fatty Liver Phenotype by Regulating Hepatic Lipid Metabolism
- PMID: 35517790
- PMCID: PMC9065278
- DOI: 10.3389/fphar.2022.850128
FGF9 Alleviates the Fatty Liver Phenotype by Regulating Hepatic Lipid Metabolism
Abstract
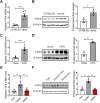
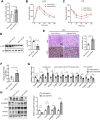
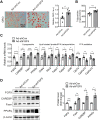
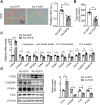
Although the fatty liver has been linked to numerous impairments of energy homeostasis, the molecular mechanism responsible for fatty liver development remains largely unknown. In the present study, we show that fibroblast growth factors 9 (FGF9) expression is increased in the liver of diet-induced obese (DIO), db/db, and ob/ob mice relative to their respective controls. The long-term knockdown of hepatic FGF9 expression mediated by adeno-associated virus expressing FGF9-specific short hairpin RNA (AAV-shFGF9) aggravated the fatty liver phenotype of DIO mice. Consistently, downregulation of FGF9 expression mediated by adenovirus expressing FGF9-specific shRNA (Ad-shFGF9) in the primary hepatocyte promoted the cellular lipid accumulation, suggesting that FGF9 exerts its effects in an autocrine manner. In contrast, adenoviruses expressing FGF9 (Ad-FGF9) mediated FGF9 overexpression in the liver of DIO mice alleviated hepatic steatosis and improved the insulin sensitivity and glucose intolerance. Moreover, the liver-specific FGF9 transgenic mice phenocopied the Ad-FGF9-infected mice. Mechanistically, FGF9 inhibited the expression of genes involved in lipogenesis and increased the expression of genes involved in fatty acid oxidation, thereby reducing cellular lipid accumulation. Thus, targeting FGF9 might be exploited to treat nonalcoholic fatty liver disease (NAFLD) and metabolic syndrome.
Keywords: FGF9; fatty acid oxidation; fatty liver; lipid synthesis; lipogenesis.
Copyright © 2022 Zhao, Zhang, Zhang, Huang, Zhang and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Mouse KLF11 regulates hepatic lipid metabolism.J Hepatol. 2013 Apr;58(4):763-70. doi: 10.1016/j.jhep.2012.11.024. Epub 2012 Nov 23. J Hepatol. 2013. PMID: 23183531
-
Mesencephalic astrocyte-derived neurotrophic factor ameliorates steatosis in HepG2 cells by regulating hepatic lipid metabolism.World J Gastroenterol. 2020 Mar 14;26(10):1029-1041. doi: 10.3748/wjg.v26.i10.1029. World J Gastroenterol. 2020. PMID: 32205994 Free PMC article.
-
Hepatic miR-192-3p reactivation alleviates steatosis by targeting glucocorticoid receptor.JHEP Rep. 2020 Sep 6;2(6):100179. doi: 10.1016/j.jhepr.2020.100179. eCollection 2020 Dec. JHEP Rep. 2020. PMID: 33134908 Free PMC article.
-
Inhibition of hepatocyte nuclear factor 1b induces hepatic steatosis through DPP4/NOX1-mediated regulation of superoxide.Free Radic Biol Med. 2017 Dec;113:71-83. doi: 10.1016/j.freeradbiomed.2017.09.016. Epub 2017 Sep 21. Free Radic Biol Med. 2017. PMID: 28942246 Free PMC article.
-
Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones.J Endocrinol. 2017 Jul;234(1):R1-R21. doi: 10.1530/JOE-16-0513. Epub 2017 Apr 20. J Endocrinol. 2017. PMID: 28428362 Review.
Cited by
-
FGF9 Recruits β-Catenin to Increase Hepatic ECM Synthesis and Promote NASH-Driven HCC.Adv Sci (Weinh). 2023 Oct;10(28):e2301166. doi: 10.1002/advs.202301166. Epub 2023 Aug 11. Adv Sci (Weinh). 2023. PMID: 37566761 Free PMC article.
-
Fgf9 regulates bone marrow mesenchymal stem cell fate and bone-fat balance in osteoporosis by PI3K/AKT/Hippo and MEK/ERK signaling.Int J Biol Sci. 2024 Jun 17;20(9):3461-3479. doi: 10.7150/ijbs.94863. eCollection 2024. Int J Biol Sci. 2024. PMID: 38993574 Free PMC article.
-
Regulation of Hair Follicle Growth and Development by Different Alternative Spliceosomes of FGF5 in Rabbits.Genes (Basel). 2024 Mar 26;15(4):409. doi: 10.3390/genes15040409. Genes (Basel). 2024. PMID: 38674344 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

