Antibody-Dependent Complement Responses toward SARS-CoV-2 Receptor-Binding Domain Immobilized on "Pseudovirus-like" Nanoparticles
- PMID: 35507641
- PMCID: PMC9092195
- DOI: 10.1021/acsnano.2c02794
Antibody-Dependent Complement Responses toward SARS-CoV-2 Receptor-Binding Domain Immobilized on "Pseudovirus-like" Nanoparticles
Abstract
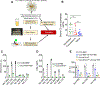
Many aspects of innate immune responses to SARS viruses remain unclear. Of particular interest is the role of emerging neutralizing antibodies against the receptor-binding domain (RBD) of SARS-CoV-2 in complement activation and opsonization. To overcome challenges with purified virions, here we introduce "pseudovirus-like" nanoparticles with ∼70 copies of functional recombinant RBD to map complement responses. Nanoparticles fix complement in an RBD-dependent manner in sera of all vaccinated, convalescent, and naı̈ve donors, but vaccinated and convalescent donors with the highest levels of anti-RBD antibodies show significantly higher IgG binding and higher deposition of the third complement protein (C3). The opsonization via anti-RBD antibodies is not an efficient process: on average, each bound antibody promotes binding of less than one C3 molecule. C3 deposition is exclusively through the alternative pathway. C3 molecules bind to protein deposits, but not IgG, on the nanoparticle surface. Lastly, "pseudovirus-like" nanoparticles promote complement-dependent uptake by granulocytes and monocytes in the blood of vaccinated donors with high anti-RBD titers. Using nanoparticles displaying SARS-CoV-2 proteins, we demonstrate subject-dependent differences in complement opsonization and immune recognition.
Keywords: SARS-CoV-2; antibody; complement; iron oxide nanoparticle; opsonization; receptor-binding domain.
Figures


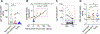
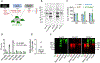

Similar articles
-
Complement opsonization of nanoparticles: Differences between humans and preclinical species.J Control Release. 2021 Oct 10;338:548-556. doi: 10.1016/j.jconrel.2021.08.048. Epub 2021 Sep 2. J Control Release. 2021. PMID: 34481928 Free PMC article.
-
A highly sensitive bead-based flow cytometric competitive binding assay to detect SARS-CoV-2 neutralizing antibody activity.Front Immunol. 2022 Nov 30;13:1041860. doi: 10.3389/fimmu.2022.1041860. eCollection 2022. Front Immunol. 2022. PMID: 36532082 Free PMC article.
-
Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice.bioRxiv [Preprint]. 2021 Jan 5:2020.11.17.387092. doi: 10.1101/2020.11.17.387092. bioRxiv. 2021. Update in: Science. 2021 Feb 12;371(6530):735-741. doi: 10.1126/science.abf6840. PMID: 33236016 Free PMC article. Updated. Preprint.
-
Correlation of the Commercial Anti-SARS-CoV-2 Receptor Binding Domain Antibody Test with the Chemiluminescent Reduction Neutralizing Test and Possible Detection of Antibodies to Emerging Variants.Microbiol Spectr. 2021 Dec 22;9(3):e0056021. doi: 10.1128/Spectrum.00560-21. Epub 2021 Dec 1. Microbiol Spectr. 2021. PMID: 34851163 Free PMC article.
-
In Vitro and In Vivo Differences in Murine Third Complement Component (C3) Opsonization and Macrophage/Leukocyte Responses to Antibody-Functionalized Iron Oxide Nanoworms.Front Immunol. 2017 Feb 15;8:151. doi: 10.3389/fimmu.2017.00151. eCollection 2017. Front Immunol. 2017. PMID: 28239384 Free PMC article.
Cited by
-
Nanometer- and angstrom-scale characteristics that modulate complement responses to nanoparticles.J Control Release. 2022 Nov;351:432-443. doi: 10.1016/j.jconrel.2022.09.039. Epub 2022 Sep 27. J Control Release. 2022. PMID: 36152807 Free PMC article.
-
Biophysical evolution of the receptor-binding domains of SARS-CoVs.Biophys J. 2023 Dec 5;122(23):4489-4502. doi: 10.1016/j.bpj.2023.10.026. Epub 2023 Oct 28. Biophys J. 2023. PMID: 37897042 Free PMC article.
-
Surface Modification of Erythrocytes with Lipid Anchors: Structure-Activity Relationship for Optimal Membrane Incorporation, in vivo Retention, and Immunocompatibility.Adv Nanobiomed Res. 2022 Aug;2(8):2200037. doi: 10.1002/anbr.202200037. Epub 2022 Jul 19. Adv Nanobiomed Res. 2022. PMID: 36591390 Free PMC article.
-
Perspectives on complement and phagocytic cell responses to nanoparticles: From fundamentals to adverse reactions.J Control Release. 2023 Apr;356:115-129. doi: 10.1016/j.jconrel.2023.02.022. Epub 2023 Mar 2. J Control Release. 2023. PMID: 36841287 Free PMC article.
References
-
- Moghimi SM; Andersen AJ; Ahmadvand D; Wibroe PP; Andresen TL; Hunter AC Material Properties in Complement Activation. Advanced Drug Delivery Reviews 2011, 63, 1000–1007. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

