Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics
- PMID: 35502223
- PMCID: PMC9045030
- DOI: 10.1007/s40778-022-00212-1
Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics
Abstract
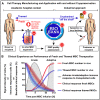
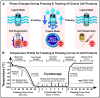
Purpose of review: Cryopreservation and its associated freezing and thawing procedures-short "freeze-thawing"-are among the final steps in economically viable manufacturing and clinical application of diverse cellular therapeutics. Translation from preclinical proof-of-concept studies to larger clinical trials has indicated that these processes may potentially present an Achilles heel to optimal cell product safety and particularly efficacy in clinical trials and routine use.
Recent findings: We review the current state of the literature on how cryopreservation of cellular therapies has evolved and how the application of this technique to different cell types is interlinked with their ability to engraft and function upon transfer in vivo, in particular for hematopoietic stem and progenitor cells (HSPCs), their progeny, and therapeutic cell products derived thereof. We also discuss pros and cons how this may differ for non-hematopoietic mesenchymal stromal/stem cell (MSC) therapeutics. We present different avenues that may be crucial for cell therapy optimization, both, for hematopoietic (e.g., effector, regulatory, and chimeric antigen receptor (CAR)-modified T and NK cell based products) and for non-hematopoietic products, such as MSCs and induced pluripotent stem cells (iPSCs), to achieve optimal viability, recovery, effective cell dose, and functionality of the cryorecovered cells.
Summary: Targeted research into optimizing the cryopreservation and freeze-thawing routines and the adjunct manufacturing process design may provide crucial advantages to increase both the safety and efficacy of cellular therapeutics in clinical use and to enable effective market deployment strategies to become economically viable and sustainable medicines.
Keywords: Cellular therapeutics; Cryopreservation; Effector T cells (Teff); Freeze-thawing; Functionality; Induced pluripotent stem cells (iPSCs); Mesenchymal Stromal/Stem Cells (MSCs); Natural killer (NK) cells; Regulatory T cells (Treg); Safety and efficacy; Stem cells (MSCs).
© The Author(s) 2022.
Conflict of interest statement
Conflict of InterestR.C. is an inventor on a pending patent application (US Patent 20190099449 A1) related to this publication. The other authors declare that they have no conflict of interest.
Figures


Similar articles
-
The Utilization of Freezing Steps in Mesenchymal Stromal Cell (MSC) Manufacturing: Potential Impact on Quality and Cell Functionality Attributes.Front Immunol. 2019 Jul 16;10:1627. doi: 10.3389/fimmu.2019.01627. eCollection 2019. Front Immunol. 2019. PMID: 31379832 Free PMC article.
-
Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy?Adv Exp Med Biol. 2016;951:77-98. doi: 10.1007/978-3-319-45457-3_7. Adv Exp Med Biol. 2016. PMID: 27837556 Review.
-
Umbilical cord tissue-derived mesenchymal stromal cells maintain immunomodulatory and angiogenic potencies after cryopreservation and subsequent thawing.Cytotherapy. 2017 Mar;19(3):360-370. doi: 10.1016/j.jcyt.2016.11.008. Epub 2016 Dec 28. Cytotherapy. 2017. PMID: 28040463
-
Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNγ Licensing.Stem Cells. 2016 Sep;34(9):2429-42. doi: 10.1002/stem.2415. Epub 2016 Jul 4. Stem Cells. 2016. PMID: 27299362 Free PMC article.
-
Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects.Cryobiology. 2015 Oct;71(2):181-97. doi: 10.1016/j.cryobiol.2015.07.003. Epub 2015 Jul 14. Cryobiology. 2015. PMID: 26186998 Review.
Cited by
-
Immune-Cell-Based Therapy for COVID-19: Current Status.Viruses. 2023 Oct 25;15(11):2148. doi: 10.3390/v15112148. Viruses. 2023. PMID: 38005826 Free PMC article. Review.
-
Industrial Biotechnology Conservation Processes: Similarities with Natural Long-Term Preservation of Biological Organisms.BioTech (Basel). 2023 Jan 31;12(1):15. doi: 10.3390/biotech12010015. BioTech (Basel). 2023. PMID: 36810442 Free PMC article.
-
Temperature Dependence of Membrane Permeability Parameters for Five Cell Types Using Nonideal Thermodynamic Assumptions to Mathematically Model Cryopreservation Protocols.J Phys Chem B. 2024 Feb 8;128(5):1139-1160. doi: 10.1021/acs.jpcb.3c04534. Epub 2024 Jan 31. J Phys Chem B. 2024. PMID: 38291962
-
Hypothermic Preservation of Adipose-Derived Mesenchymal Stromal Cells as a Viable Solution for the Storage and Distribution of Cell Therapy Products.Bioengineering (Basel). 2022 Dec 14;9(12):805. doi: 10.3390/bioengineering9120805. Bioengineering (Basel). 2022. PMID: 36551011 Free PMC article.
-
The Impact of Cryopreservation on Hematopoietic Stem Cell Engraftment and Post-transplant Outcome During the COVID-19 Pandemic.In Vivo. 2024 May-Jun;38(3):1271-1277. doi: 10.21873/invivo.13565. In Vivo. 2024. PMID: 38688614 Free PMC article.
References
-
- Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk H-D, Reinke P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front Immunol. 2020;11:1091. doi: 10.3389/fimmu.2020.01091. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
