Hepatocyte Mitochondrial Dynamics and Bioenergetics in Obesity-Related Non-Alcoholic Fatty Liver Disease
- PMID: 35501558
- PMCID: PMC9399061
- DOI: 10.1007/s13679-022-00473-1
Hepatocyte Mitochondrial Dynamics and Bioenergetics in Obesity-Related Non-Alcoholic Fatty Liver Disease
Abstract
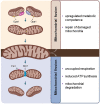
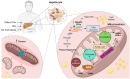
Purpose of the review: Mitochondrial dysfunction has long been proposed to play a crucial role in the pathogenesis of a considerable number of disorders, such as neurodegeneration, cancer, cardiovascular, and metabolic disorders, including obesity-related insulin resistance and non-alcoholic fatty liver disease (NAFLD). Mitochondria are highly dynamic organelles that undergo functional and structural adaptations to meet the metabolic requirements of the cell. Alterations in nutrient availability or cellular energy needs can modify their formation through biogenesis and the opposite processes of fission and fusion, the fragmentation, and connection of mitochondrial network areas respectively. Herein, we review and discuss the current literature on the significance of mitochondrial adaptations in obesity and metabolic dysregulation, emphasizing on the role of hepatocyte mitochondrial flexibility in obesity and NAFLD.
Recent findings: Accumulating evidence suggests the involvement of mitochondrial morphology and bioenergetics dysregulations to the emergence of NAFLD and its progress to non-alcoholic steatohepatitis (NASH). Most relevant data suggests that changes in liver mitochondrial dynamics and bioenergetics hold a key role in the pathogenesis of NAFLD. During obesity and NAFLD, oxidative stress occurs due to the excessive production of ROS, leading to mitochondrial dysfunction. As a result, mitochondria become incompetent and uncoupled from respiratory chain activities, further promoting hepatic fat accumulation, while leading to liver inflammation, insulin resistance, and disease's deterioration. Elucidation of the mechanisms leading to dysfunctional mitochondrial activity of the hepatocytes during NAFLD is of predominant importance for the development of novel therapeutic approaches towards the treatment of this metabolic disorder.
Keywords: Energy metabolism; Liver; Mitochondrial bioenergetics; Mitochondrial dysfunction; NAFLD; NASH.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
From Non-Alcoholic Fatty Liver to Hepatocellular Carcinoma: A Story of (Mal)Adapted Mitochondria.Biology (Basel). 2023 Apr 14;12(4):595. doi: 10.3390/biology12040595. Biology (Basel). 2023. PMID: 37106795 Free PMC article. Review.
-
Non-Alcoholic Fatty Liver Disease.Adv Exp Med Biol. 2017;960:443-467. doi: 10.1007/978-3-319-48382-5_19. Adv Exp Med Biol. 2017. PMID: 28585211 Review.
-
Oxidative Stress in NAFLD: Role of Nutrients and Food Contaminants.Biomolecules. 2020 Dec 21;10(12):1702. doi: 10.3390/biom10121702. Biomolecules. 2020. PMID: 33371482 Free PMC article. Review.
-
Hepatocyte-specific activity of TSC22D4 triggers progressive NAFLD by impairing mitochondrial function.Mol Metab. 2022 Jun;60:101487. doi: 10.1016/j.molmet.2022.101487. Epub 2022 Apr 1. Mol Metab. 2022. PMID: 35378329 Free PMC article.
-
The Role of Mitochondrial Adaptation and Metabolic Flexibility in the Pathophysiology of Obesity and Insulin Resistance: an Updated Overview.Curr Obes Rep. 2021 Sep;10(3):191-213. doi: 10.1007/s13679-021-00434-0. Epub 2021 Apr 10. Curr Obes Rep. 2021. PMID: 33840072 Review.
Cited by
-
Targeting mitochondrial quality control: new therapeutic strategies for major diseases.Mil Med Res. 2024 Aug 21;11(1):59. doi: 10.1186/s40779-024-00556-1. Mil Med Res. 2024. PMID: 39164792 Free PMC article. Review.
-
Linking nutrient sensing, mitochondrial function, and PRR immune cell signaling in liver disease.Trends Immunol. 2022 Nov;43(11):886-900. doi: 10.1016/j.it.2022.09.002. Epub 2022 Oct 7. Trends Immunol. 2022. PMID: 36216719 Free PMC article. Review.
-
A Mediterranean Diet-Oriented Intervention Rescues Impaired Blood Cell Bioenergetics in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease.Diagnostics (Basel). 2024 Sep 14;14(18):2041. doi: 10.3390/diagnostics14182041. Diagnostics (Basel). 2024. PMID: 39335721 Free PMC article.
-
Coagulation Dysfunctions in Non-Alcoholic Fatty Liver Disease-Oxidative Stress and Inflammation Relevance.Medicina (Kaunas). 2023 Sep 7;59(9):1614. doi: 10.3390/medicina59091614. Medicina (Kaunas). 2023. PMID: 37763733 Free PMC article. Review.
-
The role of mitochondrial dynamics in disease.MedComm (2020). 2023 Dec 28;4(6):e462. doi: 10.1002/mco2.462. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38156294 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

