Quinoline-based fluorescent probe for the detection and monitoring of hypochlorous acid in a rheumatoid arthritis model
- PMID: 35496887
- PMCID: PMC9041640
- DOI: 10.1039/d1ra06224g
Quinoline-based fluorescent probe for the detection and monitoring of hypochlorous acid in a rheumatoid arthritis model
Abstract
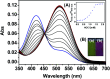
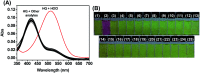
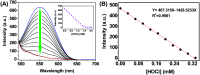
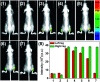
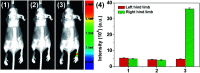
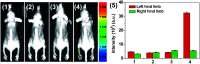
The development of effective bioanalytical methods for the visualization of hypochlorous acid (HOCl) in situ in rheumatoid arthritis (RA) directly contributes to better understanding the roles of HOCl in this disease. In this work, a new quinoline-based fluorescence probe (HQ) has been developed for the detection and visualization of a HOCl-mediated inflammatory response in a RA model. HQ possesses a donor-π-acceptor (D-π-A) structure that was designed by conjugating p-hydroxybenzaldehyde (electron donor) and 1-ethyl-4-methylquinolinium iodide (electron acceptor) through a C[double bond, length as m-dash]C double bond. In the presence of HOCl, oxidation of phenol to benzoquinone led to the red-shift (93 nm) of the adsorption and intense quenching of the fluorescence emission. The proposed response reaction mechanism was verified by high performance liquid chromatography (HPLC) and high-resolution mass spectroscopy (HRMS) titration analysis. The remarkable color changes of the HQ solution from pale yellow to pink enabled the application of HQ-stained chromatography plates for the "naked-eye" detection of HOCl in real-world water samples. HQ featured high selectivity and sensitivity (6.5 nM), fast response time (<25 s) to HOCl, reliability at different pH (3.0 to 11.5) and low cytotoxicity. HQ's application in biological systems was then demonstrated by the monitoring of HOCl-mediated treatment response to RA. This work thus provided a new tool for the detection and imaging of HOCl in inflammatory disorders.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Sequential detection of hypochlorous acid and sulfur dioxide derivatives by a red-emitting fluorescent probe and bioimaging applications in vitro and in vivo.RSC Adv. 2022 May 26;12(25):15861-15869. doi: 10.1039/d2ra01048h. eCollection 2022 May 23. RSC Adv. 2022. PMID: 35733666 Free PMC article.
-
Rapid Response Fluorescence Probe Enabled In Vivo Diagnosis and Assessing Treatment Response of Hypochlorous Acid-Mediated Rheumatoid Arthritis.Adv Sci (Weinh). 2018 Jun 7;5(8):1800397. doi: 10.1002/advs.201800397. eCollection 2018 Aug. Adv Sci (Weinh). 2018. PMID: 30128246 Free PMC article.
-
Responsive Fluorescence Probe for Selective and Sensitive Detection of Hypochlorous Acid in Live Cells and Animals.Chem Asian J. 2018 Sep 17;13(18):2611-2618. doi: 10.1002/asia.201800957. Epub 2018 Aug 6. Chem Asian J. 2018. PMID: 29963750
-
A New and Fast-Response Fluorescent Probe for Monitoring Hypochlorous Acid Derived from Myeloperoxidase.Molecules. 2023 Aug 14;28(16):6055. doi: 10.3390/molecules28166055. Molecules. 2023. PMID: 37630307 Free PMC article.
-
Imaging of Hypochlorous Acid by Fluorescence and Applications in Biological Systems.Chem Asian J. 2019 Sep 16;14(18):3048-3084. doi: 10.1002/asia.201900672. Epub 2019 Sep 2. Chem Asian J. 2019. PMID: 31347256 Review.
Cited by
-
Activatable fluorescent probes for imaging and diagnosis of rheumatoid arthritis.Mil Med Res. 2023 Jul 14;10(1):31. doi: 10.1186/s40779-023-00467-7. Mil Med Res. 2023. PMID: 37443101 Free PMC article. Review.
-
The Development of a 4-aminonaphthalimide-based Highly Selective Fluorescent Probe for Rapid Detection of HOCl.J Fluoresc. 2022 Sep;32(5):1843-1849. doi: 10.1007/s10895-022-02996-1. Epub 2022 Jun 22. J Fluoresc. 2022. PMID: 35731451
-
Sequential detection of hypochlorous acid and sulfur dioxide derivatives by a red-emitting fluorescent probe and bioimaging applications in vitro and in vivo.RSC Adv. 2022 May 26;12(25):15861-15869. doi: 10.1039/d2ra01048h. eCollection 2022 May 23. RSC Adv. 2022. PMID: 35733666 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

