Mogrol Attenuates Osteoclast Formation and Bone Resorption by Inhibiting the TRAF6/MAPK/NF-κB Signaling Pathway In vitro and Protects Against Osteoporosis in Postmenopausal Mice
- PMID: 35496311
- PMCID: PMC9038946
- DOI: 10.3389/fphar.2022.803880
Mogrol Attenuates Osteoclast Formation and Bone Resorption by Inhibiting the TRAF6/MAPK/NF-κB Signaling Pathway In vitro and Protects Against Osteoporosis in Postmenopausal Mice
Abstract
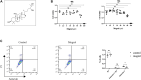
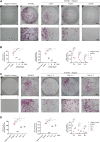
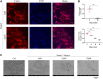
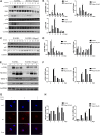
Osteoporosis is a serious public health problem that results in fragility fractures, especially in postmenopausal women. Because the current therapeutic strategy for osteoporosis has various side effects, a safer and more effective treatment is worth exploring. It is important to examine natural plant extracts during new drug design due to low toxicity. Mogrol is an aglycon of mogroside, which is the active component of Siraitia grosvenorii (Swingle) and exhibits anti-inflammatory, anticancer and neuroprotective effects. Here, we demonstrated that mogrol dose-dependently inhibited osteoclast formation and function. To confirm the mechanism, RNA sequencing (RNA-seq), real-time PCR (RT-PCR), immunofluorescence and Western blotting were performed. The RNA-seq data revealed that mogrol had an effect on genes involved in osteoclastogenesis. Furthermore, RT-PCR indicated that mogrol suppressed osteoclastogenesis-related gene expression, including CTSK, ACP5, MMP9 and DC-STAMP, in RANKL-induced bone marrow macrophages Western blotting demonstrated that mogrol suppressed osteoclast formation by blocking TNF receptor-associated factor 6 (TRAF6)-dependent activation of the mitogen-activated protein kinase nuclear factor-B (NF-κB) signaling pathway, which decreased two vital downstream transcription factors, the nuclear factor of activated T cells calcineurin-dependent 1 (NFATc1) and c-Fos proteins expression. Furthermore, mogrol dramatically reduced bone mass loss in postmenopausal mice. In conclusion, these data showed that mogrol may be a promising procedure for osteoporosis prevention or therapy.
Keywords: MAPK; NF-κB; RANKL; TRAF6; mogrol; osteoclast; osteoporosis.
Copyright © 2022 Chen, Zhang, Li, Wu, Lin, Li, Shen, Wei, Yu, Gong, Rui, Xu and Ji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases.Life (Basel). 2023 Feb 16;13(2):555. doi: 10.3390/life13020555. Life (Basel). 2023. PMID: 36836915 Free PMC article. Review.
-
Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways.J Bone Miner Res. 2012 Jun;27(6):1298-1308. doi: 10.1002/jbmr.1576. J Bone Miner Res. 2012. PMID: 22337253
-
Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-κB and PI3K/Akt signaling pathways.Bone. 2015 Jun;75:128-37. doi: 10.1016/j.bone.2015.02.017. Epub 2015 Feb 21. Bone. 2015. PMID: 25708053
-
Rosavin suppresses osteoclastogenesis in vivo and in vitro by blocking the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways.Ann Transl Med. 2021 Mar;9(5):383. doi: 10.21037/atm-20-4255. Ann Transl Med. 2021. PMID: 33842604 Free PMC article.
-
TRAF6 Mediates Suppression of Osteoclastogenesis and Prevention of Ovariectomy-Induced Bone Loss by a Novel Prenylflavonoid.J Bone Miner Res. 2017 Apr;32(4):846-860. doi: 10.1002/jbmr.3031. Epub 2017 Feb 23. J Bone Miner Res. 2017. PMID: 27813153
Cited by
-
Deciphering the protective effect of Buzhong Yiqi Decoction on osteoporotic fracture through network pharmacology and experimental validation.J Orthop Surg Res. 2023 Feb 4;18(1):86. doi: 10.1186/s13018-023-03545-7. J Orthop Surg Res. 2023. PMID: 36737821 Free PMC article.
-
Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases.Life (Basel). 2023 Feb 16;13(2):555. doi: 10.3390/life13020555. Life (Basel). 2023. PMID: 36836915 Free PMC article. Review.
-
Anti-osteoporosis activity of casticin in ovariectomized rats.Toxicol Res (Camb). 2024 Apr 26;13(2):tfae064. doi: 10.1093/toxres/tfae064. eCollection 2024 Apr. Toxicol Res (Camb). 2024. PMID: 38680951
-
Mogrol stimulates G-protein-coupled bile acid receptor 1 (GPBAR1/TGR5) and insulin secretion from pancreatic β-cells and alleviates hyperglycemia in mice.Sci Rep. 2024 Feb 8;14(1):3244. doi: 10.1038/s41598-024-53380-x. Sci Rep. 2024. PMID: 38332164 Free PMC article.
-
Recent advances of NFATc1 in rheumatoid arthritis-related bone destruction: mechanisms and potential therapeutic targets.Mol Med. 2024 Feb 3;30(1):20. doi: 10.1186/s10020-024-00788-w. Mol Med. 2024. PMID: 38310228 Free PMC article. Review.
References
-
- Beinke S., Robinson M. J., Hugunin M., Ley S. C. (2004). Lipopolysaccharide Activation of the TPL-2/MEK/extracellular Signal-Regulated Kinase Mitogen-Activated Protein Kinase cascade Is Regulated by IkappaB Kinase-Induced Proteolysis of NF-kappaB1 P105. Mol. Cel. Biol. 24, 9658–9667. 10.1128/mcb.24.21.9658-9667.2004 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

