A Novel Virus-Like Agent Originated From Genome Rearrangement of Porcine Circovirus Type 2 (PCV2) Enhances PCV2 Replication and Regulates Intracellular Redox Status In Vitro
- PMID: 35493731
- PMCID: PMC9043654
- DOI: 10.3389/fcimb.2022.855920
A Novel Virus-Like Agent Originated From Genome Rearrangement of Porcine Circovirus Type 2 (PCV2) Enhances PCV2 Replication and Regulates Intracellular Redox Status In Vitro
Abstract
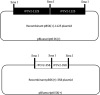
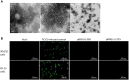
Genome rearrangement occurs to porcine circovirus type 2 (PCV2) during in vitro and in vivo infections, and a number of rearranged PCV2 genomes have been isolated and characterized. This study was conducted to investigate the role of the rearranged PCV2 (rPCV2) in PCV2 replication and the biological effect of rPCV2 in host cells. Two whole rPCV2 genome sequences (358 nt and 1125 nt in length) were synthesized and recombinant plasmids pBSK(+)-rPCV2 (pBSK(+)-1125 and pBSK(+)-358) were constructed. A novel virus-like agent (rPCV2-1125) was rescued by in vitro transfection of porcine kidney cell line (PK-15) and porcine alveolar macrophage 3D4/21 cells. The data indicate that rPCV2-1125 significantly enhanced PCV2 replication in vitro. Furthermore, rPCV2-1125 led to oxidative stress in host cells, as indicated by decreased intracellular glutathione (GSH) and total superoxide dismutase (SOD) activities, as well as increased malondialdehyde (MDA) levels. These results provide new insights into genome rearrangement of PCV2 and will contribute to future studies of PCV2 replication and associated mechanisms.
Keywords: genome rearrangement; porcine circovirus type 2; redox status; viral replication; virus-like agent.
Copyright © 2022 Feng, Fu, Zhang, Xue and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Interaction of porcine circovirus type 2 replication with intracellular redox status in vitro.Redox Rep. 2013;18(5):186-92. doi: 10.1179/1351000213Y.0000000058. Epub 2013 Jul 23. Redox Rep. 2013. PMID: 23902724 Free PMC article.
-
Vimentin promotes porcine circovirus type 2 (PCV2) replication in pig alveolar macrophage.Virus Res. 2022 Sep;318:198842. doi: 10.1016/j.virusres.2022.198842. Epub 2022 Jun 1. Virus Res. 2022. PMID: 35662567
-
Porcine IGFBP3 promotes porcine circovirus type 2 replication via PERK/eIF2α mediated DNA damage.Vet Microbiol. 2023 Dec;287:109897. doi: 10.1016/j.vetmic.2023.109897. Epub 2023 Oct 28. Vet Microbiol. 2023. PMID: 37922860
-
Genomic Rearrangement and Recombination of Porcine Circovirus Type 2 and Porcine Circovirus-Like Virus P1 in China.Front Vet Sci. 2021 Dec 20;8:736366. doi: 10.3389/fvets.2021.736366. eCollection 2021. Front Vet Sci. 2021. PMID: 34988138 Free PMC article. Review.
-
Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system.Annu Rev Anim Biosci. 2013 Jan;1:43-64. doi: 10.1146/annurev-animal-031412-103720. Epub 2013 Jan 3. Annu Rev Anim Biosci. 2013. PMID: 25387012 Review.
Cited by
-
Detection of Porcine Circovirus 1/2/3 and Genetic Analysis of Porcine Circovirus 2 in Wild Boar from Jiangxi Province of China.Animals (Basel). 2022 Aug 10;12(16):2021. doi: 10.3390/ani12162021. Animals (Basel). 2022. PMID: 36009613 Free PMC article.
References
-
- Allan G. M., McNeilly F., Ellis J., Krakowka S., Meehan B., McNair I., et al. . (2000). Experimental Infection of Colostrum Deprived Piglets With Porcine Circovirus 2 (PCV2) and Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Potentiates PCV2 Replication. Arch. Virol. 145, 2421–2429. doi: 10.1007/s007050070031 - DOI - PubMed
-
- Chang H. W., Jeng C. R., Liu J. J., Lin T. L., Chang C. C., Chia M. Y., et al. . (2005). Reduction of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Infection in Swine Alveolar Macrophages by Porcine Circovirus 2 (PCV2)-Induced Interferon-Alpha. Vet. Microbiol. 108, 167–177. doi: 10.1016/j.vetmic.2005.03.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

