Neuronal Cell Adhesion Molecules May Mediate Neuroinflammation in Autism Spectrum Disorder
- PMID: 35492721
- PMCID: PMC9051034
- DOI: 10.3389/fpsyt.2022.842755
Neuronal Cell Adhesion Molecules May Mediate Neuroinflammation in Autism Spectrum Disorder
Abstract
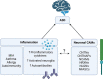
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition characterized by restrictive and repetitive behaviors, alongside deficits in social interaction and communication. The etiology of ASD is largely unknown but is strongly linked to genetic variants in neuronal cell adhesion molecules (CAMs), cell-surface proteins that have important roles in neurodevelopment. A combination of environmental and genetic factors are believed to contribute to ASD pathogenesis. Inflammation in ASD has been identified as one of these factors, demonstrated through the presence of proinflammatory cytokines, maternal immune activation, and activation of glial cells in ASD brains. Glial cells are the main source of cytokines within the brain and, therefore, their activity is vital in mediating inflammation in the central nervous system. However, it is unclear whether the aforementioned neuronal CAMs are involved in modulating neuroimmune signaling or glial behavior. This review aims to address the largely unexplored role that neuronal CAMs may play in mediating inflammatory cascades that underpin neuroinflammation in ASD, primarily focusing on the Notch, nuclear factor-κB (NF-κB), and mitogen-activated protein kinase (MAPK) cascades. We will also evaluate the available evidence on how neuronal CAMs may influence glial activity associated with inflammation. This is important when considering the impact of environmental factors and inflammatory responses on ASD development. In particular, neural CAM1 (NCAM1) can regulate NF-κB transcription in neurons, directly altering proinflammatory signaling. Additionally, NCAM1 and contactin-1 appear to mediate astrocyte and oligodendrocyte precursor proliferation which can alter the neuroimmune response. Importantly, although this review highlights the limited information available, there is evidence of a neuronal CAM regulatory role in inflammatory signaling. This warrants further investigation into the role other neuronal CAM family members may have in mediating inflammatory cascades and would advance our understanding of how neuroinflammation can contribute to ASD pathology.
Keywords: autism spectrum disorder; cell adhesion molecules; glial cells; inflammatory cascade; neuroinflammation; neuroinflammatory signaling.
Copyright © 2022 Eve, Gandawijaya, Yang and Oguro-Ando.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cell Adhesion Molecules Involved in Neurodevelopmental Pathways Implicated in 3p-Deletion Syndrome and Autism Spectrum Disorder.Front Cell Neurosci. 2021 Jan 13;14:611379. doi: 10.3389/fncel.2020.611379. eCollection 2020. Front Cell Neurosci. 2021. PMID: 33519384 Free PMC article. Review.
-
Exploring Astrocytes Involvement and Glutamate Induced Neuroinflammation in Chlorpyrifos-Induced Paradigm Of Autism Spectrum Disorders (ASD).Neurochem Res. 2024 Sep;49(9):2573-2599. doi: 10.1007/s11064-024-04191-z. Epub 2024 Jun 19. Neurochem Res. 2024. PMID: 38896196
-
Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD).Brain Behav Immun. 2023 Feb;108:245-254. doi: 10.1016/j.bbi.2022.12.001. Epub 2022 Dec 6. Brain Behav Immun. 2023. PMID: 36494048 Review.
-
Methylmercury chloride exposure aggravates proinflammatory mediators and Notch-1 signaling in CD14+ and CD40+ cells and is associated with imbalance of neuroimmune function in BTBR T+ Itpr3tf/J mice.Neurotoxicology. 2021 Jan;82:9-17. doi: 10.1016/j.neuro.2020.10.014. Epub 2020 Nov 6. Neurotoxicology. 2021. PMID: 33166615
-
Potential crosstalk between sonic hedgehog-WNT signaling and neurovascular molecules: Implications for blood-brain barrier integrity in autism spectrum disorder.J Neurochem. 2021 Oct;159(1):15-28. doi: 10.1111/jnc.15460. Epub 2021 Aug 6. J Neurochem. 2021. PMID: 34169527 Free PMC article. Review.
Cited by
-
A glia-enriched stem cell 3D model of the human brain mimics the glial-immune neurodegenerative phenotypes of multiple sclerosis.Cell Rep Med. 2024 Aug 20;5(8):101680. doi: 10.1016/j.xcrm.2024.101680. Epub 2024 Aug 8. Cell Rep Med. 2024. PMID: 39121861 Free PMC article.
-
Autoimmune nodopathy with anti-contactin 1 antibody characterized by cerebellar dysarthria: a case report and literature review.Front Immunol. 2024 Mar 8;15:1308068. doi: 10.3389/fimmu.2024.1308068. eCollection 2024. Front Immunol. 2024. PMID: 38524138 Free PMC article. Review.
-
Revealing cell-cell communication pathways with their spatially coupled gene programs.Brief Bioinform. 2024 Mar 27;25(3):bbae202. doi: 10.1093/bib/bbae202. Brief Bioinform. 2024. PMID: 38706319 Free PMC article.
-
Causal relationships between atopic dermatitis and psychiatric disorders: a bidirectional two-sample Mendelian randomization study.BMC Psychiatry. 2024 Jan 3;24(1):16. doi: 10.1186/s12888-023-05478-1. BMC Psychiatry. 2024. PMID: 38172785 Free PMC article.
-
Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein as Potential Diagnostic Biomarkers in Autism Spectrum Disorders: A Preliminary Study.Int J Mol Sci. 2023 Feb 3;24(3):3057. doi: 10.3390/ijms24033057. Int J Mol Sci. 2023. PMID: 36769380 Free PMC article.
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; (2013).
-
- Tsai LY. Impact of DSM-5 on epidemiology of autism spectrum disorder. Res Autism Spectr Disord. (2014) 8:1454–70. 10.1016/j.rasd.2014.07.016 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

