Heptanol-mediated phase separation determines phase preference of molecules in live cell membranes
- PMID: 35490741
- PMCID: PMC9160352
- DOI: 10.1016/j.jlr.2022.100220
Heptanol-mediated phase separation determines phase preference of molecules in live cell membranes
Abstract
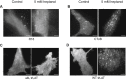
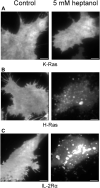
The localization of many membrane proteins within cholesterol- and sphingolipid-containing microdomains is essential for proper cell signaling and function. These membrane domains, however, are too small and dynamic to be recorded, even with modern super-resolution techniques. Therefore, the association of membrane proteins with these domains can only be detected with biochemical assays that destroy the integrity of cells require pooling of many cells and take a long time to perform. Here, we present a simple membrane fluidizer-induced clustering approach to identify the phase-preference of membrane-associated molecules in individual live cells within 10-15 min. Experiments in phase-separated bilayers and live cells on molecules with known phase preference show that heptanol hyperfluidizes the membrane and stabilizes phase separation. This results in a transition from nanosized to micronsized clusters of associated molecules allowing their identification using routine microscopy techniques. Membrane fluidizer-induced clustering is an inexpensive and easy to implement method that can be conducted at large-scale and allows easy identification of protein partitioning in live cell membranes.
Keywords: MFIC; alcohols; assay; clustering; epidermal growth factor receptor; fluidizers; heptanol; membranes; phase separation; phases.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Similar articles
-
Membrane phase separation drives responsive assembly of receptor signaling domains.Nat Chem Biol. 2023 Jun;19(6):750-758. doi: 10.1038/s41589-023-01268-8. Epub 2023 Mar 30. Nat Chem Biol. 2023. PMID: 36997644 Free PMC article.
-
Applications of phase-separating multi-bilayers in protein-membrane domain interactions.Methods Enzymol. 2024;700:275-294. doi: 10.1016/bs.mie.2024.04.024. Methods Enzymol. 2024. PMID: 38971603
-
Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies.Biochim Biophys Acta. 2005 Dec 30;1746(3):193-202. doi: 10.1016/j.bbamcr.2005.09.003. Epub 2005 Sep 23. Biochim Biophys Acta. 2005. PMID: 16271405 Review.
-
Order of lipid phases in model and plasma membranes.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16645-50. doi: 10.1073/pnas.0908987106. Epub 2009 Sep 15. Proc Natl Acad Sci U S A. 2009. PMID: 19805351 Free PMC article.
-
Seeing spots: complex phase behavior in simple membranes.Biochim Biophys Acta. 2005 Dec 30;1746(3):172-85. doi: 10.1016/j.bbamcr.2005.06.010. Epub 2005 Jul 6. Biochim Biophys Acta. 2005. PMID: 16043244 Review.
Cited by
-
Enhanced Membrane Fluidization and Cholesterol Displacement by 1-Heptanol Inhibit Mast Cell Effector Functions.Cells. 2023 Aug 15;12(16):2069. doi: 10.3390/cells12162069. Cells. 2023. PMID: 37626879 Free PMC article.
-
The dependence of EGFR oligomerization on environment and structure: A camera-based N&B study.Biophys J. 2022 Dec 6;121(23):4452-4466. doi: 10.1016/j.bpj.2022.11.003. Epub 2022 Nov 5. Biophys J. 2022. PMID: 36335429 Free PMC article.
References
-
- Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. - PubMed
-
- Lingwood D., Kaiser H.J., Levental I., Simons K. Lipid rafts as functional heterogeneity in cell membranes. Biochem. Soc. Trans. 2009;37:955–960. - PubMed
-
- Kusumi A., Suzuki K.G.N., Kasai R.S., Ritchie K., Fujiwara T.K. Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem. Sci. 2011;36:604–615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

