Immunogenicity and safety of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine in people living with and without HIV-1 infection: a randomised, controlled, phase 2A/2B trial
- PMID: 35489376
- PMCID: PMC9045746
- DOI: 10.1016/S2352-3018(22)00041-8
Immunogenicity and safety of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine in people living with and without HIV-1 infection: a randomised, controlled, phase 2A/2B trial
Abstract
Background: There is a paucity of data on COVID-19 vaccines in people living with HIV-1, who could be at increased risk of severe illness and death from COVID-19. We evaluated the safety and immunogenicity of a Matrix-M adjuvanted recombinant spike protein nanoparticle COVID-19 vaccine (NVX-CoV2373; Novavax) in HIV-negative people and people living with HIV-1.
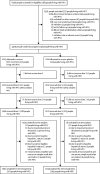
Methods: In this randomised, observer-blinded, multicentre, placebo-controlled phase 2A/B trial in South Africa, participants aged 18-84 years, with and without underlying HIV-1, were enrolled from 16 sites and randomly assigned (1:1) to receive two intramuscular injections of NVX-CoV2373 or placebo, 21 days apart. People living with HIV-1 were on stable antiretroviral therapy and had an HIV-1 viral load of less than 1000 copies per mL. Vaccine dosage was 5 μg SARS-CoV-2 recombinant spike protein with 50 μg Matrix-M adjuvant, whereas 0·9% saline was used as placebo injection (volume 0·5 mL each). All study staff and participants remained masked to study group assignment. We previously reported an interim analysis on the efficacy and safety of the NVX-CoV2373 vaccine (coprimary endpoints). In this Article, we present an expanded safety analysis for the full cohort of participants and report on the secondary objective of vaccine immunogenicity in the full cohort of people living with HIV-1 and in HIV-negative individuals overall and stratified by baseline SARS-CoV-2 serostatus. This trial is registered with ClinicalTrials.gov, NCT04533399, and the Pan-African Clinical Trials Registry, PACTR202009726132275.
Findings: Participants were enrolled between Aug 17 and Nov 25, 2020. The safety analysis set included 4164 HIV-negative participants (2089 in the intervention group and 2075 in the placebo group) and 244 people living with HIV-1 (122 in the intervention group and 122 in the placebo group). 1422 (34·1%) of 4164 HIV-negative people and 83 (34·0%) of 244 people living with HIV-1 were categorised as baseline SARS-CoV-2-positive (ie, anti-spike IgG reactive at enrolment or had a reactive SARS-CoV-2 nucleic acid amplification test by 14 days after the second study vaccination). In the NVX-CoV2373 group, solicited local and systemic adverse events were more common in HIV-negative participants (427 [30·6%] local and 401 [28·7%] systemic) than in people living with HIV-1 (20 [25·3%] local and 20 [25·3%] systemic) among those who were baseline SARS-CoV-2-seronegative (naive). Of the serious adverse events that occurred among HIV-negative people (of whom, two [0·1%] were baseline SARS-CoV-2-negative and four [0·6%] were baseline SARS-CoV-2-positive) and people living with HIV-1 (for whom there were no serious adverse events) in the NVX-CoV2373 group, none were assessed as related to the vaccine. Among participants who were baseline SARS-CoV-2-negative in the NVX-CoV2373 group, the anti-spike IgG geometric mean titres (GMTs) and seroconversion rates (SCRs) were lower in people living with HIV-1 (n=62) than in HIV-negative people (n=1234) following the first vaccination (GMT: 508·6 vs 1195·3 ELISA units [EU]/mL; SCR: 51·6% vs 81·3%); and similarly so 14 days after the second vaccination for GMTs (14 420·5 vs 31 631·8 EU/mL), whereas the SCR was similar at this point (100·0% vs 99·3%). In the NVX-CoV2373 group, anti-spike IgG GMTs 14 days after the second vaccination were substantially higher in those who were baseline SARS-CoV-2-positive than in those who were baseline SARS-CoV-2-seronegative for HIV-negative participants (100 666·1 vs 31 631·8 EU/mL) and for people living with HIV-1 (98 399·5 vs 14 420·5 EU/mL). This was also the case for angiotensin-converting enzyme 2 receptor-binding antibody and neutralising antibody titres.
Interpretation: The safety of the NVX-CoV2373 vaccine in people living with HIV-1 was similar to that in HIV-negative participants. However, people living with HIV-1 not previously exposed to SARS-CoV-2 had attenuated humoral immune responses to NVX-CoV2373 compared with their HIV-negative vaccine counterparts, but not so if they were baseline SARS-CoV-2-positive.
Funding: Novavax and the Bill & Melinda Gates Foundation; investigational vaccine manufacturing support was provided by the Coalition for Epidemic Preparedness Innovations.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests SAM reports receiving grant support, paid to his institution, from BMGF, Novavax, Pfizer, GlaxoSmithKline, and Minervax, and receiving honoraria from Sanofi for lectures unrelated to the current study. SH reports receiving grant support, paid to her institution, from DRILL, Fogarty International Center, National Insitutes of Health (NIH) Common Fund, Office of Strategic Coordination, Office of the Director, Office of AIDS Research, and the NIH National Institute of Mental Health, under award number D43TW010131. QB reports receiving grant support, paid to his institution, from Wits Health Consortium, Regeneron Pharmaceuticals, GlaxoSmithKline, Avillion, Sanofi, Novo Nordisk, the Bill & Melinda Gates Foundation, South African Medical Research Council, AstraZeneca, Clover, and Novavax. LFF reports receiving financial support from Novavax for trial procedures. LF reports receiving fees as a contractor and being a paid employee and stock shareholder of Novavax. GA reports being employed by Novavax. MZ, SN, SC-C, CB, IC, EF, AR, and VS report being employed by and owning shares in Novavax. JSP reports receiving grant support from the Bill & Melinda Gates Foundation and being employed by and owning shares in Novavax. GMG reports being employed by and owning stock in Novavax and receiving financial support from the Bill & Melinda Gates Foundation, unrelated to the current study. All other authors declare no competing interests.
Figures

Comment in
-
HIV and COVID-19: juxtaposition of two pandemics.Lancet HIV. 2022 May;9(5):e300-e301. doi: 10.1016/S2352-3018(22)00095-9. Lancet HIV. 2022. PMID: 35489372 Free PMC article. No abstract available.
Similar articles
-
Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial.Lancet Infect Dis. 2022 Nov;22(11):1565-1576. doi: 10.1016/S1473-3099(22)00420-0. Epub 2022 Aug 10. Lancet Infect Dis. 2022. PMID: 35963274 Free PMC article. Clinical Trial.
-
Immunogenicity and safety following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine with Matrix-MTM adjuvant (NVX-CoV2373) versus a primary series in people living with and without HIV-1 infection in South Africa: A randomized crossover phase 2a/2b trial.Hum Vaccin Immunother. 2024 Dec 31;20(1):2425147. doi: 10.1080/21645515.2024.2425147. Epub 2024 Dec 12. Hum Vaccin Immunother. 2024. PMID: 39666396 Free PMC article. Clinical Trial.
-
Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial.Lancet Respir Med. 2022 Feb;10(2):167-179. doi: 10.1016/S2213-2600(21)00409-4. Epub 2021 Nov 17. Lancet Respir Med. 2022. PMID: 34800364 Free PMC article. Clinical Trial.
-
Safety, efficacy, and immunogenicity of the NVX-CoV2373 vaccine.Expert Rev Vaccines. 2023 Jan-Dec;22(1):501-517. doi: 10.1080/14760584.2023.2218913. Expert Rev Vaccines. 2023. PMID: 37246757 Review.
-
Comparative efficacy and safety of COVID-19 vaccines in phase III trials: a network meta-analysis.BMC Infect Dis. 2024 Feb 21;24(1):234. doi: 10.1186/s12879-023-08754-3. BMC Infect Dis. 2024. PMID: 38383356 Free PMC article. Review.
Cited by
-
Immunogenicity and Safety of Heterologous Omicron BA.1 and Bivalent SARS-CoV-2 Recombinant Spike Protein Booster Vaccines: A Phase 3 Randomized Clinical Trial.J Infect Dis. 2024 Jul 25;230(1):e4-e16. doi: 10.1093/infdis/jiad508. J Infect Dis. 2024. PMID: 39052718 Free PMC article. Clinical Trial.
-
Safety and Immunogenicity of an Accelerated Ebola Vaccination Schedule in People with and without Human Immunodeficiency Virus: A Randomized Clinical Trial.Vaccines (Basel). 2024 May 4;12(5):497. doi: 10.3390/vaccines12050497. Vaccines (Basel). 2024. PMID: 38793748 Free PMC article.
-
Safety and immunogenicity of ChAdOx1 nCoV-19 (AZD1222) vaccine in adults in Kenya: a phase 1/2 single-blind, randomised controlled trial.Wellcome Open Res. 2023 Nov 27;8:182. doi: 10.12688/wellcomeopenres.19150.2. eCollection 2023. Wellcome Open Res. 2023. PMID: 38707489 Free PMC article.
-
Immunogenicity and Safety of SARS-CoV-2 Protein Subunit Recombinant Vaccine (IndoVac®) as a Booster Dose against COVID-19 in Indonesian Adults.Vaccines (Basel). 2024 May 14;12(5):540. doi: 10.3390/vaccines12050540. Vaccines (Basel). 2024. PMID: 38793791 Free PMC article.
-
Frequently Asked Questions on Coronavirus Disease 2019 Vaccination for Hematopoietic Cell Transplantation and Chimeric Antigen Receptor T-Cell Recipients From the American Society for Transplantation and Cellular Therapy and the American Society of Hematology.Transplant Cell Ther. 2023 Jan;29(1):10-18. doi: 10.1016/j.jtct.2022.10.010. Epub 2022 Oct 21. Transplant Cell Ther. 2023. PMID: 36273782 Free PMC article.
References
-
- UNAIDS Fact sheet—World Aids Day 2021. Nov 29, 2021. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

