The return of the rings: Evolutionary convergence of aromatic residues in the intrinsically disordered regions of RNA-binding proteins for liquid-liquid phase separation
- PMID: 35481633
- PMCID: PMC9045073
- DOI: 10.1002/pro.4317
The return of the rings: Evolutionary convergence of aromatic residues in the intrinsically disordered regions of RNA-binding proteins for liquid-liquid phase separation
Abstract
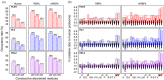
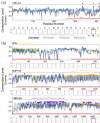
Aromatic residues appeared relatively late in the evolution of protein sequences to stabilize the globular proteins' folding core and are less in the intrinsically disordered regions (IDRs). Recent advances in protein liquid-liquid phase separation (LLPS) studies have also shown that aromatic residues in IDRs often act as "stickers" to promote multivalent interactions in forming higher-order oligomers. To study how general these structure-promoting residues are in IDRs, we compared levels of sequence disorder in RNA binding proteins (RBPs), which are often found to undergo LLPS, and the human proteome. We found that aromatic residues appear more frequently than expected in the IDRs of RBPs and, through multiple sequence alignment analysis, those aromatic residues are often conserved among chordates. Using TDP-43, FUS, and some other well-studied LLPS proteins as examples, the conserved aromatic residues are important to their LLPS-related functions. These analyses suggest that aromatic residues may have contributed twice to evolution: stabilizing structured proteins and assembling biomolecular condensates.
Keywords: RNA-binding proteins; biomolecular condensates; intrinsically disordered proteins; liquid-liquid phase separation; membraneless organelle.
© 2022 The Protein Society.
Figures
Similar articles
-
TAR DNA-binding protein 43 (TDP-43) liquid-liquid phase separation is mediated by just a few aromatic residues.J Biol Chem. 2018 Apr 20;293(16):6090-6098. doi: 10.1074/jbc.AC117.001037. Epub 2018 Mar 6. J Biol Chem. 2018. PMID: 29511089 Free PMC article.
-
Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs.J Biol Chem. 2017 Nov 17;292(46):19110-19120. doi: 10.1074/jbc.M117.800466. Epub 2017 Sep 18. J Biol Chem. 2017. PMID: 28924037 Free PMC article.
-
Interactions between the Intrinsically Disordered Regions of hnRNP-A2 and TDP-43 Accelerate TDP-43's Conformational Transition.Int J Mol Sci. 2020 Aug 18;21(16):5930. doi: 10.3390/ijms21165930. Int J Mol Sci. 2020. PMID: 32824743 Free PMC article.
-
How do RNA binding proteins trigger liquid-liquid phase separation in human health and diseases?Biosci Trends. 2022 Dec 26;16(6):389-404. doi: 10.5582/bst.2022.01449. Epub 2022 Dec 3. Biosci Trends. 2022. PMID: 36464283 Review.
-
Adenosine Triphosphate: The Primordial Molecule That Controls Protein Homeostasis and Shapes the Genome-Proteome Interface.Biomolecules. 2024 Apr 19;14(4):500. doi: 10.3390/biom14040500. Biomolecules. 2024. PMID: 38672516 Free PMC article. Review.
Cited by
-
Programmable de novo designed coiled coil-mediated phase separation in mammalian cells.Nat Commun. 2023 Dec 2;14(1):7973. doi: 10.1038/s41467-023-43742-w. Nat Commun. 2023. PMID: 38042897 Free PMC article.
-
Modulation of biophysical properties of nucleocapsid protein in the mutant spectrum of SARS-CoV-2.Elife. 2024 Jun 28;13:RP94836. doi: 10.7554/eLife.94836. Elife. 2024. PMID: 38941236 Free PMC article.
-
Modulation of Biophysical Properties of Nucleocapsid Protein in the Mutant Spectrum of SARS-CoV-2.bioRxiv [Preprint]. 2024 Mar 22:2023.11.21.568093. doi: 10.1101/2023.11.21.568093. bioRxiv. 2024. Update in: Elife. 2024 Jun 28;13:RP94836. doi: 10.7554/eLife.94836 PMID: 38045241 Free PMC article. Updated. Preprint.
-
TDP-43 Amyloid Fibril Formation via Phase Separation-Related and -Unrelated Pathways.ACS Chem Neurosci. 2024 Oct 2;15(20):3767-75. doi: 10.1021/acschemneuro.4c00503. Online ahead of print. ACS Chem Neurosci. 2024. PMID: 39358890 Free PMC article.
-
A Few Charged Residues in Galectin-3's Folded and Disordered Regions Regulate Phase Separation.Adv Sci (Weinh). 2024 Nov;11(41):e2402570. doi: 10.1002/advs.202402570. Epub 2024 Sep 9. Adv Sci (Weinh). 2024. PMID: 39248370 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

