The timing of differentiation and potency of CD8 effector function is set by RNA binding proteins
- PMID: 35477960
- PMCID: PMC9046422
- DOI: 10.1038/s41467-022-29979-x
The timing of differentiation and potency of CD8 effector function is set by RNA binding proteins
Abstract
CD8+ T cell differentiation into effector cells is initiated early after antigen encounter by signals from the T cell antigen receptor and costimulatory molecules. The molecular mechanisms that establish the timing and rate of differentiation however are not defined. Here we show that the RNA binding proteins (RBP) ZFP36 and ZFP36L1 limit the rate of differentiation of activated naïve CD8+ T cells and the potency of the resulting cytotoxic lymphocytes. The RBP function in an early and short temporal window to enforce dependency on costimulation via CD28 for full T cell activation and effector differentiation by directly binding mRNA of NF-κB, Irf8 and Notch1 transcription factors and cytokines, including Il2. Their absence in T cells, or the adoptive transfer of small numbers of CD8+ T cells lacking the RBP, promotes resilience to influenza A virus infection without immunopathology. These findings highlight ZFP36 and ZFP36L1 as nodes for the integration of the early T cell activation signals controlling the speed and quality of the CD8+ T cell response.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


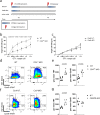
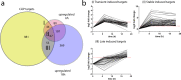

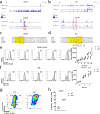
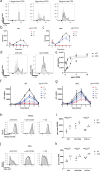
Similar articles
-
4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy.J Immunol. 2007 Oct 1;179(7):4910-8. doi: 10.4049/jimmunol.179.7.4910. J Immunol. 2007. PMID: 17878391 Free PMC article.
-
NKG2D receptor activation of NF-κB enhances inflammatory cytokine production in murine effector CD8(+) T cells.Mol Immunol. 2015 Feb;63(2):268-78. doi: 10.1016/j.molimm.2014.07.015. Epub 2014 Jul 31. Mol Immunol. 2015. PMID: 25089028
-
CD28, IL-2-independent costimulatory pathways for CD8 T lymphocyte activation.J Immunol. 1999 Aug 1;163(3):1133-42. J Immunol. 1999. PMID: 10415007
-
The role of CD40 ligand in costimulation and T-cell activation.Immunol Rev. 1996 Oct;153:85-106. doi: 10.1111/j.1600-065x.1996.tb00921.x. Immunol Rev. 1996. PMID: 9010720 Review.
-
CD28 and CD27 costimulation of CD8+ T cells: a story of survival.Adv Exp Med Biol. 2007;590:149-70. doi: 10.1007/978-0-387-34814-8_11. Adv Exp Med Biol. 2007. PMID: 17191384 Review.
Cited by
-
Time-dependent regulation of cytokine production by RNA binding proteins defines T cell effector function.Cell Rep. 2023 May 30;42(5):112419. doi: 10.1016/j.celrep.2023.112419. Epub 2023 Apr 18. Cell Rep. 2023. PMID: 37074914 Free PMC article.
-
Regulation of CD8 T Cell Differentiation by the RNA-Binding Protein DDX5.J Immunol. 2023 Jul 15;211(2):241-251. doi: 10.4049/jimmunol.2200778. J Immunol. 2023. PMID: 37265401 Free PMC article.
-
Integrative analysis of neuroblastoma by single-cell RNA sequencing identifies the NECTIN2-TIGIT axis as a target for immunotherapy.Cancer Cell. 2024 Feb 12;42(2):283-300.e8. doi: 10.1016/j.ccell.2023.12.008. Epub 2024 Jan 4. Cancer Cell. 2024. PMID: 38181797 Free PMC article.
-
Cytotoxic T lymphocytes require transcription for infiltration but not target cell lysis.EMBO Rep. 2023 Nov 6;24(11):e57653. doi: 10.15252/embr.202357653. Epub 2023 Oct 20. EMBO Rep. 2023. PMID: 37860838 Free PMC article.
-
The ZFP36 family of RNA binding proteins regulates homeostatic and autoreactive T cell responses.Sci Immunol. 2022 Oct 28;7(76):eabo0981. doi: 10.1126/sciimmunol.abo0981. Epub 2022 Oct 21. Sci Immunol. 2022. PMID: 36269839 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- MR/N010434/1/MRC_/Medical Research Council/United Kingdom
- BBS/E/B/000C0407/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/000C0428/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 200823/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- BBS/E/B/000C0427/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

