Immune Responses to SARS-CoV-2 Vaccination in Young Patients with Anti-CD19 Chimeric Antigen Receptor T Cell-Induced B Cell Aplasia
- PMID: 35472554
- PMCID: PMC9040419
- DOI: 10.1016/j.jtct.2022.04.017
Immune Responses to SARS-CoV-2 Vaccination in Young Patients with Anti-CD19 Chimeric Antigen Receptor T Cell-Induced B Cell Aplasia
Abstract
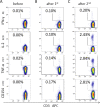
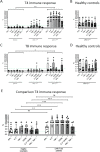
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines are capable of inducing combined humoral and cellular immunity. Which effect is more relevant for their potent protective effects is unclear, but isolated T cell responses without seroconversion in healthy household members of individuals with Coronavirus disease 19 (COVID-19) suggest that T cell responses effectively protect against clinical infection. Oncologic patients have an outsize risk of unfavorable outcomes after SARS-CoV-2 infection and therefore were prioritized when vaccines first became available, although the quality of their immune response to vaccination was expected to be suboptimal, as has been confirmed in subsequent studies. Inherently, patients with anti-CD19 chimeric antigen receptor (CAR) T cell therapy-mediated B cell aplasia would be incapable of generating humoral responses, so that assessment of the vaccine-induced cellular immunity is all the more important to gauge whether the vaccine can induce meaningful protection. A salient difference between T cell and humoral responses is the former's relative impassiveness to mutations of the antigen, which is more relevant than ever since the advent of the omicron variant. The objective of this study was to assess the immune cell composition and spike protein-specific T cell responses before and after the first and second doses of SARS-CoV-2 mRNA vaccine in a cohort of juvenile CD19 CAR T cell therapy recipients with enduring B cell aplasia. The prospective study included all patients age >12 years diagnosed with multiply relapsed B cell precursor acute lymphoblastic leukemia and treated with anti-CD19 CAR T cell (CAR-T19) therapy in our center. The primary endpoint was the detection of cell-mediated and humoral responses to vaccine (flow cytometry and anti-S immunoglobulin G, respectively). Secondary endpoints included the incidence of vaccine-related grade 3 or 4 adverse events, exacerbation of graft-versus-host disease (GVHD), relapse, and the influence of the vaccine on CAR T cells and lymphocyte subsets. Even though one-half of the patients exhibited subnormal lymphocyte counts and marginal CD4/CD8 ratios, after 2 vaccinations all showed brisk T-cell responsiveness to spike protein, predominantly in the CD4 compartment, which quantitatively was well within the range of healthy controls. No severe vaccine-related grade 3 or 4 adverse events, GVHD exacerbation, or relapse was observed in our cohort. We posit that SARS-CoV-2 mRNA vaccines induce meaningful cellular immunity in patients with isolated B cell deficiency due to CAR-T19 therapy.
Keywords: Anti-CD19 CAR T cell-induced B cell aplasia; CAR T cell therapy; Immune response; SARS-CoV-2 vaccination.
Copyright © 2022 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures

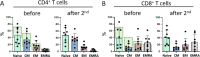
Comment in
-
COVID-19 Vaccination and Anti-CD19 CAR T Cell-Induced B Cell Aplasia.Transplant Cell Ther. 2022 Aug;28(8):515. doi: 10.1016/j.jtct.2022.05.024. Epub 2022 May 19. Transplant Cell Ther. 2022. PMID: 35598839 Free PMC article. No abstract available.
Similar articles
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Immunogenicity among Chimeric Antigen Receptor T Cell Therapy Recipients.Transplant Cell Ther. 2023 Jun;29(6):398.e1-398.e5. doi: 10.1016/j.jtct.2023.03.005. Epub 2023 Mar 9. Transplant Cell Ther. 2023. PMID: 36906276 Free PMC article.
-
Specific immune response to mRNA vaccines against COVID-19 in patients receiving allogeneic stem cell transplantation for myeloid malignancy was altered by immunosuppressive therapy.Leuk Res. 2023 Jul;130:107314. doi: 10.1016/j.leukres.2023.107314. Epub 2023 May 16. Leuk Res. 2023. PMID: 37216792 Free PMC article.
-
State of the CAR-T: Risk of Infections with Chimeric Antigen Receptor T-Cell Therapy and Determinants of SARS-CoV-2 Vaccine Responses.Transplant Cell Ther. 2021 Dec;27(12):973-987. doi: 10.1016/j.jtct.2021.09.016. Epub 2021 Sep 27. Transplant Cell Ther. 2021. PMID: 34587552 Free PMC article. Review.
-
Chimeric Antigen Receptor T Cell Therapy for Pediatric B-ALL: Narrowing the Gap Between Early and Long-Term Outcomes.Front Immunol. 2020 Aug 11;11:1985. doi: 10.3389/fimmu.2020.01985. eCollection 2020. Front Immunol. 2020. PMID: 32849662 Free PMC article. Review.
Cited by
-
Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL.Hematology Am Soc Hematol Educ Program. 2023 Dec 8;2023(1):77-83. doi: 10.1182/hematology.2023000422. Hematology Am Soc Hematol Educ Program. 2023. PMID: 38066902 Free PMC article. Review.
-
Current understanding and management of CAR T cell-associated toxicities.Nat Rev Clin Oncol. 2024 Jul;21(7):501-521. doi: 10.1038/s41571-024-00903-0. Epub 2024 May 20. Nat Rev Clin Oncol. 2024. PMID: 38769449 Free PMC article. Review.
-
Cellular and humoral responses after second and third SARS-CoV-2 vaccinations in patients with autoimmune diseases treated with rituximab: specific T cell immunity remains longer and plays a protective role against SARS-CoV-2 reinfections.Front Immunol. 2023 Apr 27;14:1146841. doi: 10.3389/fimmu.2023.1146841. eCollection 2023. Front Immunol. 2023. PMID: 37180097 Free PMC article.
-
Omicron related COVID-19 prevention and treatment measures for patients with hematological malignancy and strategies for modifying hematologic treatment regimes.Front Cell Infect Microbiol. 2023 Oct 19;13:1207225. doi: 10.3389/fcimb.2023.1207225. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37928188 Free PMC article. Review.
-
Comprehensive snapshots of natural killer cells functions, signaling, molecular mechanisms and clinical utilization.Signal Transduct Target Ther. 2024 Nov 8;9(1):302. doi: 10.1038/s41392-024-02005-w. Signal Transduct Target Ther. 2024. PMID: 39511139 Free PMC article. Review.
References
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

