Uptake of hepatitis C virus treatment in a multi-state Medicaid population, 2013-2017
- PMID: 35466398
- PMCID: PMC9643082
- DOI: 10.1111/1475-6773.13994
Uptake of hepatitis C virus treatment in a multi-state Medicaid population, 2013-2017
Abstract
Objective: To examine trends in the direct acting antiviral (DAA) uptake in a multi-state Medicaid population with hepatitis C virus (HCV) prior to and after ledipasvir/sofosbuvir (LDV/SOF) approval and changes in prior authorization (PA) requirements.
Data sources: Analyses utilized enrollment, medical, and pharmacy claims in four states, December 2013-December 2017.
Study design: An interrupted time series examined trends in uptake (1+ claim for a DAA) before and after two events: LDV/SOV approval (October 2014) and lifting of PA requirements for 40% of members (July 2016). Analyses were also performed in subgroups defined by the number and dates of change in PA requirements in members' Medicaid plans.
Data collection/extraction methods: Members aged 18-64 years with an ICD code for HCV were included in the sample from diagnosis date until treatment initiation or Medicaid disenrollment.
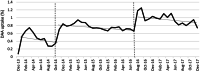
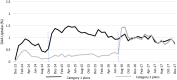
Principal findings: The annual sample size ranged from 38,302 to 45,005 with approximately 30% ages 18-34 years and 40% female. In December 2013, 0.08% was treated, rising to 0.74% in December 2017 (p < 0.001). Uptake increased from 0.34%/month in October 2014 to 0.70%/month after LDV/SOF approval, (p < 0.001), and increased relative to the pre-LDV/SOV trend through June 2016 (p = 0.04). Uptake increased to 1.18%/month after PA change, (p < 0.001) and remained flat through 2017 (p = 0.64). Cumulatively, 20.1% were treated by December 2017. In plans with few/no requirements through 2017, uptake increased to 1.19%/month after LDV/SOF approval (p < 0.001) and remained flat through 2017 (p = 0.11), with 22.2% cumulatively treated. Among plans that lifted PA requirements from three to zero in mid-2016, uptake did not increase after LDV/SOF approval (p = 0.36) but did increase to 1.41%/month (p < 0.001) after PA change, with 18.1% cumulatively treated.
Conclusions: HCV Treatment increased through 2017. LDV/SOF approval and lifting PA requirements led to an increase in uptake followed by flat monthly utilization. Cumulative uptake was higher in plans with few/no PA requirements relative to those with three requirements through mid-2016.
Keywords: Medicaid; hepatitis C virus; pharmaceuticals: prescribing/use/costs; state health policies; time-series analysis.
© 2022 Health Research and Educational Trust.
Figures
Similar articles
-
Access to New Medications for Hepatitis C for Medicaid Members: A Retrospective Cohort Study.J Manag Care Spec Pharm. 2016 Jun;22(6):714-722b. doi: 10.18553/jmcp.2016.22.6.714. J Manag Care Spec Pharm. 2016. PMID: 27231798 Free PMC article.
-
Multicenter Experience using Ledipasvir/Sofosbuvir ± RBV to Treat HCV GT 1 Relapsers after Simeprevir and Sofosbuvir Treatment.Ann Hepatol. 2018 Aug 24;17(5):815-821. doi: 10.5604/01.3001.0012.3142. Ann Hepatol. 2018. PMID: 30145562
-
Cost-Effectiveness Analysis of Pan-Genotypic Sofosbuvir-Based Regimens for Treatment of Chronic Hepatitis C Genotype 1 Infection in China.Front Public Health. 2021 Dec 9;9:779215. doi: 10.3389/fpubh.2021.779215. eCollection 2021. Front Public Health. 2021. PMID: 34957030 Free PMC article.
-
Fixed-dose combination of sofosbuvir and ledipasvir for the treatment of chronic hepatitis C genotype 1.Expert Opin Pharmacother. 2015 Apr;16(5):739-48. doi: 10.1517/14656566.2015.1013938. Epub 2015 Feb 13. Expert Opin Pharmacother. 2015. PMID: 25676581 Review.
-
Efficacy of Second Generation Direct-Acting Antiviral Agents for Treatment Naïve Hepatitis C Genotype 1: A Systematic Review and Network Meta-Analysis.PLoS One. 2015 Dec 31;10(12):e0145953. doi: 10.1371/journal.pone.0145953. eCollection 2015. PLoS One. 2015. PMID: 26720298 Free PMC article. Review.
Cited by
-
Changes in Use of Hepatitis C Direct-Acting Antivirals After Access Restrictions Were Eased by State Medicaid Programs.JAMA Health Forum. 2024 Apr 5;5(4):e240302. doi: 10.1001/jamahealthforum.2024.0302. JAMA Health Forum. 2024. PMID: 38578628 Free PMC article.
References
-
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 Suppl):S58‐S68. - PubMed
-
- Roebuck MC, Liberman JN. Assessing the burden of illness of chronic hepatitis C and impact of direct‐acting antiviral use on healthcare costs in Medicaid. Am J Manag Care. 2019;25(8 Suppl):S131‐S139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

