Isolation and Characterization of a Lytic Vibriophage OY1 and Its Biocontrol Effects Against Vibrio spp
- PMID: 35464931
- PMCID: PMC9022663
- DOI: 10.3389/fmicb.2022.830692
Isolation and Characterization of a Lytic Vibriophage OY1 and Its Biocontrol Effects Against Vibrio spp
Abstract
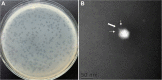
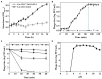
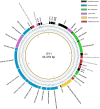
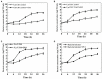
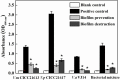
Vibrio species are important pathogens of marine animals and aquaculture populations and some of them can cause serious infections in humans through consumption of contaminated seafood and aquaculture products. Lytic bacteriophages can potentially alleviate Vibrio contamination in the aquaculture organisms and in the processing of aquatic products and have gained significant scientific attention in recent years. In the present study, bacteriophages were isolated from sewage of local aquatic products markets and grown using Vibrio mimicus CICC 21613 as host cells. The lytic vibriophage OY1 belonging to the newly proposed family Autographiviridae and the genus Maculvirus was identified by observation under electron microscope and comparative genomic analysis. The phage OY1 showed lytic activity against 24 among 32 tested strains belonging to eight Vibrio species. The complete phage OY1 genome consists of a single circular double-stranded DNA of 43,479 bp with a total GC content of 49.27% and was predicted to encode 40 open reading frames (ORFs). To evaluate its potential against vibrios, the one-step growth curve, thermal and pH stability, host range, and lytic activity of the OY1 phage against Vibrio species were evaluated. The results showed that phage OY1 had a range of thermal and pH tolerance, and exhibited a significant inhibitory effect on the growth of tested Vibrio species. Bacterial growth in the fish muscle extract juice (FMEJ) inoculated with Vibrio mimicus CICC 21613, Vibrio parahaemolyticus CICC 21617, Vibrio alginolyticus VJ14, and the mixed bacterial culture was reduced by 2.65 log CFU/ml, 2.42 log CFU/ml, 1.93 log CFU/ml, and 2.01 log CFU/ml, respectively, by incubation with phage OY1 at 25°C for 36 h. Phage OY1 also showed a strong ability to prevent biofilm formation and destroy formed Vibrio species biofilms. These results indicate that phage OY1 is a potential biocontrol agent against Vibrio species in the aquaculture industry and in food safety control.
Keywords: Vibrio species; biocontrol; biofilm; lytic activity; vibriophage.
Copyright © 2022 Gao, Ouyang, Li, Zhang, Zheng, Li, Rao, Yang and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Isolation and characterization of a novel phage belonging to a new genus against Vibrio parahaemolyticus.Virol J. 2023 May 1;20(1):81. doi: 10.1186/s12985-023-02036-9. Virol J. 2023. PMID: 37127579 Free PMC article.
-
Isolation and Characterization of a Lytic Vibrio parahaemolyticus Phage vB_VpaP_GHSM17 from Sewage Samples.Viruses. 2022 Jul 22;14(8):1601. doi: 10.3390/v14081601. Viruses. 2022. PMID: 35893666 Free PMC article.
-
Genome Characterization and Infectivity Potential of Vibriophage-ϕLV6 with Lytic Activity against Luminescent Vibrios of Penaeus vannamei Shrimp Aquaculture.Viruses. 2023 Mar 28;15(4):868. doi: 10.3390/v15040868. Viruses. 2023. PMID: 37112848 Free PMC article.
-
Phage Endolysins as Potential Antimicrobials against Multidrug Resistant Vibrio alginolyticus and Vibrio parahaemolyticus: Current Status of Research and Challenges Ahead.Microorganisms. 2019 Mar 18;7(3):84. doi: 10.3390/microorganisms7030084. Microorganisms. 2019. PMID: 30889831 Free PMC article. Review.
-
A Critical Analysis of the Opportunities and Challenges of Phage Application in Seafood Quality Control.Foods. 2024 Oct 16;13(20):3282. doi: 10.3390/foods13203282. Foods. 2024. PMID: 39456344 Free PMC article. Review.
Cited by
-
Isolation and characterization of a Vibrio owensii phage phi50-12.Sci Rep. 2022 Sep 30;12(1):16390. doi: 10.1038/s41598-022-20831-2. Sci Rep. 2022. PMID: 36180722 Free PMC article.
-
Immobilization of a broad host range phage on the peroxidase-like Fe-MOF for colorimetric determination of multiple Salmonella enterica strains in food.Mikrochim Acta. 2024 May 15;191(6):331. doi: 10.1007/s00604-024-06402-4. Mikrochim Acta. 2024. PMID: 38744722
-
Characterization and efficacy against carbapenem-resistant Acinetobacter baumannii of a novel Friunavirus phage from sewage.Front Cell Infect Microbiol. 2024 Apr 22;14:1382145. doi: 10.3389/fcimb.2024.1382145. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38736748 Free PMC article.
-
Autographiviridae phage HH109 uses capsular polysaccharide for infection of Vibrio alginolyticus.iScience. 2024 Aug 8;27(9):110695. doi: 10.1016/j.isci.2024.110695. eCollection 2024 Sep 20. iScience. 2024. PMID: 39252973 Free PMC article.
-
Vibrio-infecting bacteriophages and their potential to control biofilm.Food Sci Biotechnol. 2023 Jun 19;32(12):1719-1727. doi: 10.1007/s10068-023-01361-7. eCollection 2023 Oct. Food Sci Biotechnol. 2023. PMID: 37780594 Free PMC article. Review.
References
-
- Abedon S. T. (2021). “Detection of bacteriophages: Phage plaques,” in Bacteriophages. Biology, Technology, Therapy, 507–538.
LinkOut - more resources
Full Text Sources
Miscellaneous

