Concurrent RAS and RAS/BRAF V600E Variants in Colorectal Cancer: More Frequent Than Expected? A Case Report
- PMID: 35463316
- PMCID: PMC9022079
- DOI: 10.3389/fonc.2022.863639
Concurrent RAS and RAS/BRAF V600E Variants in Colorectal Cancer: More Frequent Than Expected? A Case Report
Abstract
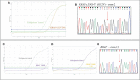
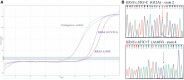
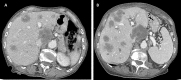
The assessment of RAS and BRAF mutational status is one of the main steps in the diagnostic and therapeutic algorithm of metastatic colorectal cancer (mCRC). Multiple mutations in the BRAF and RAS pathway are described as a rare event, with concurrent variants in KRAS and BRAF genes observed in approximately 0.05% of mCRC cases. Here, we report data from a case series affected by high-risk stage III and stage IV CRC and tested for RAS and BRAF mutation, treated at our Medical Oncology Unit. The analysis of KRAS, NRAS (codons 12, 13, 59, 61, 117, 146), and BRAF (codon 600) hotspot variants was performed in 161 CRC tumors from August 2018 to September 2021 and revealed three (1.8%) patients showing mutations in both KRAS and BRAF (V600E), including two cases with earlier CRC and one with metastatic disease. We also identified one patient (0.6%) with a mutation in both KRAS and NRAS genes and another one (0.6%) with a double KRAS mutation. Notably, the latter was characterized by aggressive behavior and poor clinical outcome. The mutational status, pathological features, and clinical history of these five CRC cases are described. Overall, this study case series adds evidence to the limited available literature concerning both the epidemiological and clinical aspects of CRC cases characterized by the presence of concurrent RAS/BRAF variants. Future multicentric studies will be required to increase the sample size and provide additional value to results observed so far in order to improve clinical management of this subgroup of CRC patients.
Keywords: case report; clinical–pathological features; colorectal cancer; concurrent RAS/BRAF variants; mutation frequency.
Copyright © 2022 Zelli, Parisi, Patruno, Cannita, Ficorella, Luzi, Compagnoni, Zazzeroni, Alesse and Tessitore.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Clinical and prognostic features of patients with detailed RAS/BRAF-mutant colorectal cancer in Japan.BMC Cancer. 2021 May 7;21(1):518. doi: 10.1186/s12885-021-08271-z. BMC Cancer. 2021. PMID: 33962575 Free PMC article.
-
Tumor profiling of KRAS, BRAF, and NRAS gene mutations in patients with colorectal cancer: A Lebanese major center cohort study.Gene. 2022 Aug 5;834:146646. doi: 10.1016/j.gene.2022.146646. Epub 2022 Jun 6. Gene. 2022. PMID: 35680020
-
Recommendations from the EGAPP Working Group: can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy?Genet Med. 2013 Jul;15(7):517-27. doi: 10.1038/gim.2012.184. Epub 2013 Feb 21. Genet Med. 2013. PMID: 23429431
-
The prognostic value of KRAS and BRAF in stage I-III colorectal cancer. A systematic review.Ann Ital Chir. 2019;90:127-137. Ann Ital Chir. 2019. PMID: 30739887
-
Immunohistochemistry with Anti-BRAF V600E (VE1) Mouse Monoclonal Antibody is a Sensitive Method for Detection of the BRAF V600E Mutation in Colon Cancer: Evaluation of 120 Cases with and without KRAS Mutation and Literature Review.Pathol Oncol Res. 2019 Jan;25(1):349-359. doi: 10.1007/s12253-017-0344-x. Epub 2017 Nov 10. Pathol Oncol Res. 2019. PMID: 29127628 Free PMC article. Review.
Cited by
-
Colorectal Cancer Brain Metastasis With Concomitant KRAS and BRAF Mutations: A Case Report.Cureus. 2024 Sep 9;16(9):e68975. doi: 10.7759/cureus.68975. eCollection 2024 Sep. Cureus. 2024. PMID: 39385899 Free PMC article.
-
KRAS, NRAS and BRAF Mutational Profile of Colorectal Cancer in a Series of Moroccan Patients.Cancer Control. 2024 Jan-Dec;31:10732748241262179. doi: 10.1177/10732748241262179. Cancer Control. 2024. PMID: 38875469 Free PMC article.
-
Follicular cell-derived thyroid carcinomas harboring novel genetic BRAFNON-V600E mutations: real-world data obtained using a multigene panel.Arch Endocrinol Metab. 2024 Sep 17;68:e240067. doi: 10.20945/2359-4292-2024-0067. eCollection 2024. Arch Endocrinol Metab. 2024. PMID: 39420942 Free PMC article.
References
-
- Larki P, Gharib E, Yaghoob Taleghani M, Khorshidi F, Nazemalhosseini-Mojarad E, Asadzadeh Aghdaei H. Coexistence of KRAS and BRAF Mutations in Colorectal Cancer: A Case Report Supporting The Concept of Tumoral Heterogeneity. Cell J (2017) 19(Suppl 1):113–7. doi: 10.22074/cellj.2017.5123 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

