AXL regulates neuregulin1 expression leading to cetuximab resistance in head and neck cancer
- PMID: 35461210
- PMCID: PMC9035247
- DOI: 10.1186/s12885-022-09511-6
AXL regulates neuregulin1 expression leading to cetuximab resistance in head and neck cancer
Abstract
Background: The receptor tyrosine kinase (RTK) epidermal growth factor receptor (EGFR) is overexpressed and an important therapeutic target in Head and Neck cancer (HNC). Cetuximab is currently the only EGFR-targeting agent approved by the FDA for treatment of HNC; however, intrinsic and acquired resistance to cetuximab is a major problem in the clinic. Our lab previously reported that AXL leads to cetuximab resistance via activation of HER3. In this study, we investigate the connection between AXL, HER3, and neuregulin1 (NRG1) gene expression with a focus on understanding how their interdependent signaling promotes resistance to cetuximab in HNC.
Methods: Plasmid or siRNA transfections and cell-based assays were conducted to test cetuximab sensitivity. Quantitative PCR and immunoblot analysis were used to analyze gene and protein expression levels. Seven HNC patient-derived xenografts (PDXs) were evaluated for protein expression levels.
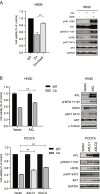
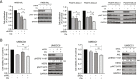
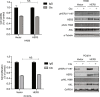
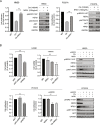
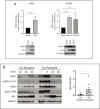
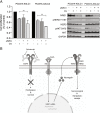
Results: We found that HER3 expression was necessary but not sufficient for cetuximab resistance without AXL expression. Our results demonstrated that addition of the HER3 ligand NRG1 to cetuximab-sensitive HNC cells leads to cetuximab resistance. Further, AXL-overexpressing cells regulate NRG1 at the level of transcription, thereby promoting cetuximab resistance. Immunoblot analysis revealed that NRG1 expression was relatively high in cetuximab-resistant HNC PDXs compared to cetuximab-sensitive HNC PDXs. Finally, genetic inhibition of NRG1 resensitized AXL-overexpressing cells to cetuximab.
Conclusions: The results of this study indicate that AXL may signal through HER3 via NRG1 to promote cetuximab resistance and that targeting of NRG1 could have significant clinical implications for HNC therapeutic approaches.
Keywords: AXL; Cetuximab; HER3; Head and neck cancer; Resistance; neuregulin1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
Similar articles
-
RETRACTED: The receptor tyrosine kinase AXL mediates nuclear translocation of the epidermal growth factor receptor.Sci Signal. 2017 Jan 3;10(460):eaag1064. doi: 10.1126/scisignal.aag1064. Sci Signal. 2017. Retraction in: Sci Signal. 2021 Nov 09;14(708):eabn0168. doi: 10.1126/scisignal.abn0168. PMID: 28049763 Free PMC article. Retracted.
-
AXL Mediates Cetuximab and Radiation Resistance Through Tyrosine 821 and the c-ABL Kinase Pathway in Head and Neck Cancer.Clin Cancer Res. 2020 Aug 15;26(16):4349-4359. doi: 10.1158/1078-0432.CCR-19-3142. Epub 2020 May 21. Clin Cancer Res. 2020. PMID: 32439698 Free PMC article.
-
AXL mediates resistance to cetuximab therapy.Cancer Res. 2014 Sep 15;74(18):5152-64. doi: 10.1158/0008-5472.CAN-14-0294. Epub 2014 Aug 18. Cancer Res. 2014. PMID: 25136066 Free PMC article.
-
Human epidermal growth factor receptor 3 in head and neck squamous cell carcinomas.Head Neck. 2016 Apr;38 Suppl 1:E2412-8. doi: 10.1002/hed.24367. Epub 2016 Feb 2. Head Neck. 2016. PMID: 26835877 Review.
-
Molecular Pathways: AXL, a Membrane Receptor Mediator of Resistance to Therapy.Clin Cancer Res. 2016 Mar 15;22(6):1313-7. doi: 10.1158/1078-0432.CCR-15-1458. Epub 2016 Jan 13. Clin Cancer Res. 2016. PMID: 26763248 Free PMC article. Review.
Cited by
-
Decoding the research landscape of drug resistance and therapeutic approaches in head and neck cancer: a bibliometric analysis from 2000 to 2023.Front Pharmacol. 2024 Apr 5;15:1375110. doi: 10.3389/fphar.2024.1375110. eCollection 2024. Front Pharmacol. 2024. PMID: 38645557 Free PMC article.
-
A Receptor Tyrosine Kinase Inhibitor Sensitivity Prediction Model Identifies AXL Dependency in Leukemia.Int J Mol Sci. 2023 Feb 14;24(4):3830. doi: 10.3390/ijms24043830. Int J Mol Sci. 2023. PMID: 36835239 Free PMC article.
-
Targeting HER3-dependent activation of nuclear AKT improves radiotherapy of non-small cell lung cancer.Radiother Oncol. 2022 Sep;174:92-100. doi: 10.1016/j.radonc.2022.07.008. Epub 2022 Jul 15. Radiother Oncol. 2022. PMID: 35839938 Free PMC article.
-
AXL in cancer: a modulator of drug resistance and therapeutic target.J Exp Clin Cancer Res. 2023 Jun 16;42(1):148. doi: 10.1186/s13046-023-02726-w. J Exp Clin Cancer Res. 2023. PMID: 37328828 Free PMC article. Review.
-
Disrupting EGFR-HER2 Transactivation by Pertuzumab in HER2-Positive Cancer: Quantitative Analysis Reveals EGFR Signal Input as Potential Predictor of Therapeutic Outcome.Int J Mol Sci. 2024 May 29;25(11):5978. doi: 10.3390/ijms25115978. Int J Mol Sci. 2024. PMID: 38892166 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- McDaniel NK, Fischbach SR, Ondracek OJ, Welke NB, Iida M, Wheeler DL. TAM family protein and therapy resistance. In: Kimple RJ, editor. Improving the therapeutic ratio in head and neck cancer. Oxford: Elsevier; 2020. Vol. 6. p. 159–192. ISBN:978-0-12-817868-3.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

