Preclinical Therapeutic Assessment of a New Chemotherapeutics [Dichloro(4,4'-Bis(2,2,3,3-Tetrafluoropropoxy) Methyl)-2,2'-Bipryridine) Platinum] in an Orthotopic Patient-Derived Xenograft Model of Triple-Negative Breast Cancers
- PMID: 35456673
- PMCID: PMC9031226
- DOI: 10.3390/pharmaceutics14040839
Preclinical Therapeutic Assessment of a New Chemotherapeutics [Dichloro(4,4'-Bis(2,2,3,3-Tetrafluoropropoxy) Methyl)-2,2'-Bipryridine) Platinum] in an Orthotopic Patient-Derived Xenograft Model of Triple-Negative Breast Cancers
Abstract
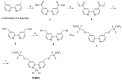
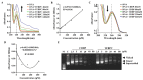
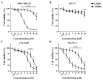
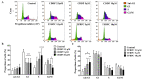
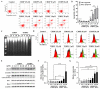
Cisplatin is one of the most common therapeutics used in treatments of several types of cancers. To enhance cisplatin lipophilicity and reduce resistance and side effects, a polyfluorinated bipyridine-modified cisplatin analogue, dichloro[4,4'-bis(2,2,3,3-tetrafluoropropoxy)methyl)-2,2'-bipryridine] platinum (TFBPC), was synthesized and therapeutic assessments were performed. TFBPC displayed superior effects in inhibiting the proliferation of several cisplatin-resistant human cancer cell lines, including MDA-MB-231 breast cancers, COLO205 colon cancers and SK-OV-3 ovarian cancers. TFBPC bound to DNA and formed DNA crosslinks that resulted in DNA degradation, triggering the cell death program through the PARP/Bax/Bcl-2 apoptosis and LC3-related autophagy pathway. Moreover, TFBPC significantly inhibited tumor growth in both animal models which include a cell line-derived xenograft model (CDX) of cisplatin-resistant MDA-MB-231, and a patient-derived xenograft (PDX) model of triple-negative breast cancers (TNBCs). Furthermore, the biopsy specimen from TFBPC-treated xenografts revealed decreased expressions of P53, Ki-67 and PD-L1 coupled with higher expression of cleaved caspase 3, suggesting TFBPC treatment was effective and resulted in good prognostic indications. No significant pathological changes were observed in hematological and biochemistry tests in blood and histological examinations from the specimen of major organs. Therefore, TFBPC is a potential candidate for treatments of patients suffering from TNBCs as well as other cisplatin-resistant cancers.
Keywords: PD-L1; apoptosis; autophagy; cell line-derived xenograft model; cisplatin; cisplatin-resistant; patient-derived xenograft; triple-negative breast cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
CARP-1 functional mimetics are novel inhibitors of drug-resistant triple negative breast cancers.Oncotarget. 2016 Nov 8;7(45):73370-73388. doi: 10.18632/oncotarget.12333. Oncotarget. 2016. PMID: 27687593 Free PMC article.
-
Simultaneous Multi-Organ Metastases from Chemo-Resistant Triple-Negative Breast Cancer Are Prevented by Interfering with WNT-Signaling.Cancers (Basel). 2019 Dec 17;11(12):2039. doi: 10.3390/cancers11122039. Cancers (Basel). 2019. PMID: 31861131 Free PMC article.
-
Inhibition of ROS production, autophagy or apoptosis signaling reversed the anticancer properties of Antrodia salmonea in triple-negative breast cancer (MDA-MB-231) cells.Food Chem Toxicol. 2017 May;103:1-17. doi: 10.1016/j.fct.2017.02.019. Epub 2017 Feb 20. Food Chem Toxicol. 2017. PMID: 28219700
-
Adeno-associated virus type 2 infection of nude mouse human breast cancer xenograft induces necrotic death and inhibits tumor growth.Cancer Biol Ther. 2014 Aug;15(8):1013-28. doi: 10.4161/cbt.29172. Epub 2014 May 16. Cancer Biol Ther. 2014. PMID: 24834917 Free PMC article.
-
Molecular, Cellular, and Technical Aspects of Breast Cancer Cell Lines as a Foundational Tool in Cancer Research.Life (Basel). 2023 Dec 8;13(12):2311. doi: 10.3390/life13122311. Life (Basel). 2023. PMID: 38137912 Free PMC article. Review.
Cited by
-
Engineering H2O2 Self-Supplying Platform for Xdynamic Therapies via Ru-Cu Peroxide Nanocarrier: Tumor Microenvironment-Mediated Synergistic Therapy.ACS Appl Mater Interfaces. 2024 May 15;16(19):24172-24190. doi: 10.1021/acsami.3c18888. Epub 2024 Apr 30. ACS Appl Mater Interfaces. 2024. PMID: 38688027 Free PMC article.
References
Grants and funding
- Ministry of Science and Technology, Taiwan/Ministry of Science and Technology, Taiwan
- MOST108-2320-B-038-033-MY3/Ministry of Science and Technology, Taiwan
- 110-2320-B-038-082 awarded/Ministry of Science and Technology, Taiwan
- MOST109-2314-B-038-033-MY3/Ministry of Science and Technology, Taiwan
- MOHW110-TDU-B-212-144014/Health and Welfare Surcharge of Tobacco Products
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

