A Theranostic Nanocomplex Combining with Magnetic Hyperthermia for Enhanced Accumulation and Efficacy of pH-Triggering Polymeric Cisplatin(IV) Prodrugs
- PMID: 35455477
- PMCID: PMC9025582
- DOI: 10.3390/ph15040480
A Theranostic Nanocomplex Combining with Magnetic Hyperthermia for Enhanced Accumulation and Efficacy of pH-Triggering Polymeric Cisplatin(IV) Prodrugs
Abstract
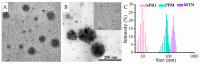
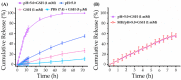
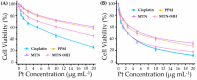
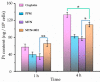
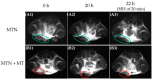
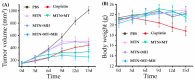
Although polymeric platinum(IV) (Pt(IV)) prodrugs can reduce the side effects of cisplatin, the efficacy of the prodrug is still limited by its non-targeted distribution, poor penetration in deep tumor tissue, and low cytotoxicity in tumor cells. To improve the clinical potential of polymeric prodrug micelle, we synthesized amphiphilic polymeric Pt(IV) with high Pt content (22.5%), then developed a theranostic nanocomplex by integrating polymeric Pt(IV) with superparamagnetic Mn0.6Zn0.4Fe2O4 via simple self-assembly. Due to the high content of Mn0.6Zn0.4Fe2O4 (41.7% w/w), the theranostic nanocomplex showed high saturation magnetization (103.1 emu g-1) and excellent magnetocaloric effect (404 W g-1), both of them indicating its advantages in efficient magnetic targeting (MT), magnetic hyperthermia (MH), and magnetic resonance imaging (MRI). In vitro, in combination with MH, the theranostic nanocomplex showed as high cytotoxicity as cisplatin because of a significant increase in platinum of cellular uptake. In vivo, the accumulation of theranostic nanocomplex in tumors was increased by MT and confirmed by MRI. Furthermore, MH improved penetration of theranostic nanocomplex in tumors as expanding blackened area in tumors was observed by MRI. Based on these properties, the theranostic nanocomplex, under the assistance of MT and MH, showed the highest tumor growth inhibition rate (88.38%) after different treatments, while the body weight of mice increased slightly, indicating low side effects compared to those of cisplatin. The study provided an advanced theranostic nanocomplex with low toxicity and high efficacy, indicating a great clinical potential of polymeric Pt(IV).
Keywords: magnetic hyperthermia; magnetic resonance imaging; magnetic targeting; pH-triggering releasing; polymeric prodrug; theranostic nanocomplex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Enhanced tumor penetration for efficient chemotherapy by a magnetothermally sensitive micelle combined with magnetic targeting and magnetic hyperthermia.Front Pharmacol. 2022 Nov 18;13:1045976. doi: 10.3389/fphar.2022.1045976. eCollection 2022. Front Pharmacol. 2022. PMID: 36467035 Free PMC article.
-
A theranostic prodrug delivery system based on Pt(IV) conjugated nano-graphene oxide with synergistic effect to enhance the therapeutic efficacy of Pt drug.Biomaterials. 2015 May;51:12-21. doi: 10.1016/j.biomaterials.2015.01.074. Epub 2015 Feb 14. Biomaterials. 2015. PMID: 25770993
-
Enhanced magnetic fluid hyperthermia by micellar magnetic nanoclusters composed of Mn(x)Zn(1-x)Fe(2)O(4) nanoparticles for induced tumor cell apoptosis.ACS Appl Mater Interfaces. 2014 Oct 8;6(19):16867-79. doi: 10.1021/am5042934. Epub 2014 Sep 24. ACS Appl Mater Interfaces. 2014. PMID: 25204363
-
Recent Approaches to Platinum(IV) Prodrugs: A Variety of Strategies for Enhanced Delivery and Efficacy.Curr Pharm Des. 2017;23(16):2366-2376. doi: 10.2174/1381612823666170201161037. Curr Pharm Des. 2017. PMID: 28155621 Review.
-
Platinum Drug-Incorporating Polymeric Nanosystems for Precise Cancer Therapy.Small. 2023 May;19(21):e2208241. doi: 10.1002/smll.202208241. Epub 2023 Feb 26. Small. 2023. PMID: 36843317 Review.
Cited by
-
Magnetic Micellar Nanovehicles: Prospects of Multifunctional Hybrid Systems for Precision Theranostics.Int J Mol Sci. 2022 Oct 4;23(19):11793. doi: 10.3390/ijms231911793. Int J Mol Sci. 2022. PMID: 36233094 Free PMC article. Review.
-
Enhanced tumor penetration for efficient chemotherapy by a magnetothermally sensitive micelle combined with magnetic targeting and magnetic hyperthermia.Front Pharmacol. 2022 Nov 18;13:1045976. doi: 10.3389/fphar.2022.1045976. eCollection 2022. Front Pharmacol. 2022. PMID: 36467035 Free PMC article.
-
Electrospun Magnetic Nanofiber Mats for Magnetic Hyperthermia in Cancer Treatment Applications-Technology, Mechanism, and Materials.Polymers (Basel). 2023 Apr 15;15(8):1902. doi: 10.3390/polym15081902. Polymers (Basel). 2023. PMID: 37112049 Free PMC article. Review.
-
Designing Highly Efficient Temperature Controller for Nanoparticles Hyperthermia.Nanomaterials (Basel). 2022 Oct 10;12(19):3539. doi: 10.3390/nano12193539. Nanomaterials (Basel). 2022. PMID: 36234672 Free PMC article.
-
Tumor Microenvironment-Responsive Magnetic Nanofluid for Enhanced Tumor MRI and Tumor multi-treatments.Pharmaceuticals (Basel). 2023 Jan 23;16(2):166. doi: 10.3390/ph16020166. Pharmaceuticals (Basel). 2023. PMID: 37259316 Free PMC article.
References
-
- Xiao H., Yan B., Dempsey E.M., Song W., Qi R., Li W., Huang Y., Jing X., Zhou D., Ding J., et al. Recent progress in polymer-based platinum drug delivery systems. Prog. Polym. Sci. 2018;87:70–106. doi: 10.1016/j.progpolymsci.2018.07.004. - DOI
LinkOut - more resources
Full Text Sources

