Design, Synthesis, and Biological Evaluation of Novel Tomentosin Derivatives in NMDA-Induced Excitotoxicity
- PMID: 35455419
- PMCID: PMC9027110
- DOI: 10.3390/ph15040421
Design, Synthesis, and Biological Evaluation of Novel Tomentosin Derivatives in NMDA-Induced Excitotoxicity
Abstract
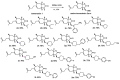
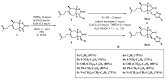
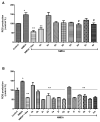
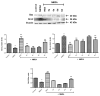
N-methyl-D-aspartate (NMDA) receptor stimulation may lead to excitotoxicity, which triggers neuronal death in brain disorders. In addition to current clinical therapeutic approaches, treatment strategies by phytochemicals or their derivatives are under investigation for neurodegenerative diseases. In the present study, novel amino and 1,2,3-triazole derivatives of tomentosin were prepared and tested for their protective and anti-apoptotic effects in NMDA-induced excitotoxicity. Amino-tomentosin derivatives were generated through a diastereoselective conjugate addition of several secondary amines to the α-methylene-γ-butyrolactone function, while the 1,2,3-triazolo-tomentosin was prepared by a regioselective Michael-type addition carried out in the presence of trimethylsilyl azide (TMSN3) and the α-methylene-γ-lactone function. The intermediate key thus obtained underwent 1,3-dipolar Huisgen cycloaddition using a wide range of terminal alkynes. The possible effects of the derivatives on cell viability and free-radical production following NMDA treatment were measured by Water-Soluble Tetrazolium Salts (WST-1) and Dichlorofluorescein Diacetate (DCF-DA) assays, respectively. The alterations in apoptosis-related proteins were examined by Western blot technique. Our study provides evidence that synthesized triazolo- and amino-tomentosin derivatives show neuroprotective effects by increasing cellular viability, decreasing ROS production, and increasing the Bcl-2/Bax ratio in NMDA-induced excitotoxicity. The findings highlight particularly 2e, 2g, and 6d as potential regulators and neuroprotective agents in NMDA overactivation.
Keywords: 1,2,3-triazolo-tomentosin; amino-tomentosin; apoptosis; excitotoxicity; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Tomentosin induces apoptotic pathway by blocking inflammatory mediators via modulation of cell proteins in AGS gastric cancer cell line.J Biochem Mol Toxicol. 2020 Aug;34(8):e22501. doi: 10.1002/jbt.22501. Epub 2020 Mar 30. J Biochem Mol Toxicol. 2020. PMID: 32227673
-
Tomentosin Displays Anti-Carcinogenic Effect in Human Osteosarcoma MG-63 Cells via the Induction of Intracellular Reactive Oxygen Species.Int J Mol Sci. 2019 Mar 26;20(6):1508. doi: 10.3390/ijms20061508. Int J Mol Sci. 2019. PMID: 30917517 Free PMC article.
-
Synthesis and Biological Evaluation of Novel Multi-target-Directed Benzazepines Against Excitotoxicity.Mol Neurobiol. 2017 Nov;54(9):6697-6722. doi: 10.1007/s12035-016-0184-9. Epub 2016 Oct 15. Mol Neurobiol. 2017. PMID: 27744571
-
Inflammatory mediators leading to protein misfolding and uncompetitive/fast off-rate drug therapy for neurodegenerative disorders.Int Rev Neurobiol. 2007;82:1-27. doi: 10.1016/S0074-7742(07)82001-0. Int Rev Neurobiol. 2007. PMID: 17678953 Review.
-
Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation.Curr Drug Targets. 2007 May;8(5):621-32. doi: 10.2174/138945007780618472. Curr Drug Targets. 2007. PMID: 17504105 Review.
References
-
- Mendez M.F., McMurtray A.M. Neurodegenerative disorders. In: Fink G., McEwen B., De Kloet E.R., Rubin R., Chrousos G., Steptoe A., Rose N., Craig I., editors. Encyclopedia of Stress. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2007.
LinkOut - more resources
Full Text Sources
Research Materials

