Hydration Dynamics of Model Peptides with Different Hydrophobic Character
- PMID: 35455063
- PMCID: PMC9031890
- DOI: 10.3390/life12040572
Hydration Dynamics of Model Peptides with Different Hydrophobic Character
Abstract
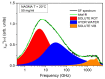
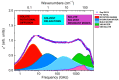
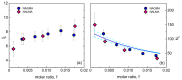
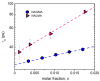
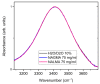
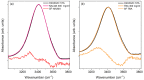
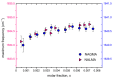
The multi-scale dynamics of aqueous solutions of the hydrophilic peptide N-acetyl-glycine-methylamide (NAGMA) have been investigated through extended frequency-range depolarized light scattering (EDLS), which enables the broad-band detection of collective polarizability anisotropy fluctuations. The results have been compared to those obtained for N-acetyl-leucinemethylamide (NALMA), an amphiphilic peptide which shares with NAGMA the same polar backbone, but also contains an apolar group. Our study indicates that the two model peptides induce similar effects on the fast translational dynamics of surrounding water. Both systems slow down the mobility of solvating water molecules by a factor 6-8, with respect to the bulk. Moreover, the two peptides cause a comparable far-reaching spatial perturbation extending to more than two hydration layers in diluted conditions. The observed concentration dependence of the hydration number is explained considering the random superposition of different hydration shells, while no indication of solute aggregation phenomena has been found. The results indicate that the effect on the dynamics of water solvating the amphiphilic peptide is dominated by the hydrophilic backbone. The minor impact of the hydrophobic moiety on hydration features is consistent with structural findings derived by Fourier transform infrared (FTIR) measurements, performed in attenuated total reflectance (ATR) configuration. Additionally, we give evidence that, for both systems, the relaxation mode in the GHz frequency range probed by EDLS is related to solute rotational dynamics. The rotation of NALMA occurs at higher timescales, with respect to the rotation of NAGMA; both processes are significantly slower than the structural dynamics of hydration water, suggesting that solute and solvent motions are uncoupled. Finally, our results do not indicate the presence of super-slow water (relaxation times in the order of tens of picoseconds) around the peptides investigated.
Keywords: ATR-FTIR; depolarized Rayleigh scattering; hydration shell; protein hydration; solvation dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Extended frequency range depolarized light scattering study of N-acetyl-leucine-methylamide-water solutions.J Am Chem Soc. 2011 Aug 10;133(31):12063-8. doi: 10.1021/ja202272k. Epub 2011 Jul 18. J Am Chem Soc. 2011. PMID: 21699224
-
Molecular view of water dynamics near model peptides.J Phys Chem B. 2005 Jul 7;109(26):12966-75. doi: 10.1021/jp051137k. J Phys Chem B. 2005. PMID: 16852609 Free PMC article.
-
Aqueous solvation of amphiphilic molecules by extended depolarized light scattering: the case of trimethylamine-N-oxide.Phys Chem Chem Phys. 2016 Apr 7;18(13):8881-9. doi: 10.1039/c5cp04357c. Phys Chem Chem Phys. 2016. PMID: 26958663
-
Depolarized light scattering and dielectric response of a peptide dissolved in water.J Chem Phys. 2014 Jan 21;140(3):035101. doi: 10.1063/1.4861965. J Chem Phys. 2014. PMID: 25669413
-
The diluted aqueous solvation of carbohydrates as inferred from molecular dynamics simulations and NMR spectroscopy.Biophys Chem. 2001 Nov 28;93(2-3):103-27. doi: 10.1016/s0301-4622(01)00215-0. Biophys Chem. 2001. PMID: 11804720 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

