Targeting pancreatic cancer with combinatorial treatment of CPI-613 and inhibitors of lactate metabolism
- PMID: 35452495
- PMCID: PMC9032382
- DOI: 10.1371/journal.pone.0266601
Targeting pancreatic cancer with combinatorial treatment of CPI-613 and inhibitors of lactate metabolism
Abstract
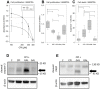
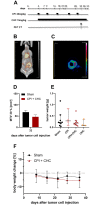
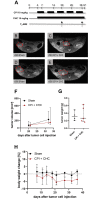
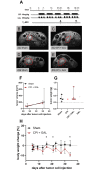
Pancreatic cancer is the fourth leading cause of cancer death, with a 5-year survival rate of 10%. A stagnant high mortality rate over the last decades highlights the need for innovative therapeutic approaches. Pancreatic tumors pursue an altered metabolism in order to maintain energy generation under low nutrient influx and hypoxic conditions. Targeting these metabolic strategies might therefore be a reasonable therapeutic approach for pancreatic cancer. One promising agent is CPI- 613, a potent inhibitor of two enzymes of the tricarboxylic acid cycle. The present study evaluated the anti-cancerous efficacy of CPI-613 in combination with galloflavin, a lactate dehydrogenase inhibitor or with alpha-cyano-4-hydroxycinnamic acid, an inhibitor of monocarboxylate transporters. The efficacy of both combination therapies was tested in vitro on one human and two murine pancreatic cancer cell lines and in vivo in an orthotopic pancreatic cancer model. Tumor progression was evaluated by MRI and 18F-FDG PET-CT. Both combinatorial treatments demonstrated in vitro a significant inhibition of pancreatic cancer cell proliferation and induction of cell death. In contrast to the in vitro results, both combination therapies did not significantly reduce tumor growth in vivo. The in vitro results suggest that a combined inhibition of different metabolic pathways might be a promising approach for cancer therapy. However, the in vivo experiments indicate that applying a higher dosage or using other drugs targeting these metabolic pathways might be more promising.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures

Similar articles
-
Galloflavin Plus Metformin Treatment Impairs Pancreatic Cancer Cells.Anticancer Res. 2020 Jan;40(1):153-160. doi: 10.21873/anticanres.13936. Anticancer Res. 2020. PMID: 31892563
-
CPI-613 rewires lipid metabolism to enhance pancreatic cancer apoptosis via the AMPK-ACC signaling.J Exp Clin Cancer Res. 2020 Apr 28;39(1):73. doi: 10.1186/s13046-020-01579-x. J Exp Clin Cancer Res. 2020. PMID: 32345326 Free PMC article.
-
Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial.Lancet Oncol. 2017 Jun;18(6):770-778. doi: 10.1016/S1470-2045(17)30314-5. Epub 2017 May 8. Lancet Oncol. 2017. PMID: 28495639 Free PMC article. Clinical Trial.
-
Monocarboxylate transporters in cancer.Mol Metab. 2020 Mar;33:48-66. doi: 10.1016/j.molmet.2019.07.006. Epub 2019 Jul 27. Mol Metab. 2020. PMID: 31395464 Free PMC article. Review.
-
Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis.Curr Pharm Des. 2012;18(10):1319-30. doi: 10.2174/138161212799504902. Curr Pharm Des. 2012. PMID: 22360558 Review.
Cited by
-
Targeting mitochondrial metabolism with CPI-613 in chemoresistant ovarian tumors.J Ovarian Res. 2024 Nov 14;17(1):226. doi: 10.1186/s13048-024-01546-6. J Ovarian Res. 2024. PMID: 39543742 Free PMC article.
-
Understanding the Contribution of Lactate Metabolism in Cancer Progress: A Perspective from Isomers.Cancers (Basel). 2022 Dec 23;15(1):87. doi: 10.3390/cancers15010087. Cancers (Basel). 2022. PMID: 36612084 Free PMC article. Review.
-
Re-Shaping the Pancreatic Cancer Tumor Microenvironment: A New Role for the Metastasis Suppressor NDRG1.Cancers (Basel). 2023 May 16;15(10):2779. doi: 10.3390/cancers15102779. Cancers (Basel). 2023. PMID: 37345116 Free PMC article. Review.
-
Sex Matters-Insights from Testing Drug Efficacy in an Animal Model of Pancreatic Cancer.Cancers (Basel). 2024 May 16;16(10):1901. doi: 10.3390/cancers16101901. Cancers (Basel). 2024. PMID: 38791980 Free PMC article.
-
The role of metabolic reprogramming in pancreatic cancer chemoresistance.Front Pharmacol. 2023 Jan 9;13:1108776. doi: 10.3389/fphar.2022.1108776. eCollection 2022. Front Pharmacol. 2023. PMID: 36699061 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

