Mitochondria and Other Organelles in Neural Development and Their Potential as Therapeutic Targets in Neurodegenerative Diseases
- PMID: 35450015
- PMCID: PMC9016280
- DOI: 10.3389/fnins.2022.853911
Mitochondria and Other Organelles in Neural Development and Their Potential as Therapeutic Targets in Neurodegenerative Diseases
Abstract
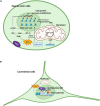
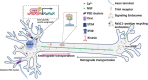
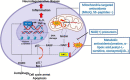
The contribution of organelles to neural development has received increasing attention. Studies have shown that organelles such as mitochondria, endoplasmic reticulum (ER), lysosomes, and endosomes play important roles in neurogenesis. Specifically, metabolic switching, reactive oxygen species production, mitochondrial dynamics, mitophagy, mitochondria-mediated apoptosis, and the interaction between mitochondria and the ER all have roles in neurogenesis. Lysosomes and endosomes can regulate neurite growth and extension. Moreover, metabolic reprogramming represents a novel strategy for generating functional neurons. Accordingly, the exploration and application of mechanisms underlying metabolic reprogramming will be beneficial for neural conversion and regenerative medicine. There is adequate evidence implicating the dysfunction of cellular organelles-especially mitochondria-in neurodegenerative disorders, and that improvement of mitochondrial function may reverse the progression of these diseases through the reinforcement of adult neurogenesis. Therefore, these organelles have potential as therapeutic targets for the treatment of neurodegenerative diseases. In this review, we discuss the function of these organelles, especially mitochondria, in neural development, focusing on their potential as therapeutic targets in neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis.
Keywords: antioxidants; metabolic reprogramming; mitochondira; neural development; neurodegenerative disease.
Copyright © 2022 Zhang, Zhao, Quan, Li and Qing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Connection Lost, MAM: Errors in ER-Mitochondria Connections in Neurodegenerative Diseases.Brain Sci. 2021 Oct 28;11(11):1437. doi: 10.3390/brainsci11111437. Brain Sci. 2021. PMID: 34827436 Free PMC article. Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Mitophagy in Alzheimer's Disease and Other Age-Related Neurodegenerative Diseases.Cells. 2020 Jan 8;9(1):150. doi: 10.3390/cells9010150. Cells. 2020. PMID: 31936292 Free PMC article. Review.
-
Defective PTEN-induced kinase 1/Parkin mediated mitophagy and neurodegenerative diseases.Front Cell Neurosci. 2022 Oct 20;16:1031153. doi: 10.3389/fncel.2022.1031153. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36339819 Free PMC article. Review.
-
Abnormalities of Mitochondrial Dynamics in Neurodegenerative Diseases.Antioxidants (Basel). 2017 Apr 5;6(2):25. doi: 10.3390/antiox6020025. Antioxidants (Basel). 2017. PMID: 28379197 Free PMC article. Review.
Cited by
-
Mitochondrial Inherited Disorders and their Correlation with Neurodegenerative Diseases.Endocr Metab Immune Disord Drug Targets. 2024;24(4):381-393. doi: 10.2174/0118715303250271231018103202. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 37937560 Review.
-
Tethering ATG16L1 or LC3 induces targeted autophagic degradation of protein aggregates and mitochondria.Autophagy. 2023 Nov;19(11):2997-3013. doi: 10.1080/15548627.2023.2234797. Epub 2023 Jul 13. Autophagy. 2023. PMID: 37424101 Free PMC article.
-
TET3 is a positive regulator of mitochondrial respiration in Neuro2A cells.PLoS One. 2024 Jan 16;19(1):e0294187. doi: 10.1371/journal.pone.0294187. eCollection 2024. PLoS One. 2024. PMID: 38227585 Free PMC article.
-
Monitoring Alzheimer's disease via ultraweak photon emission.iScience. 2023 Dec 14;27(1):108744. doi: 10.1016/j.isci.2023.108744. eCollection 2024 Jan 19. iScience. 2023. PMID: 38235338 Free PMC article.
-
Biochemical Pathways of Cellular Mechanosensing/Mechanotransduction and Their Role in Neurodegenerative Diseases Pathogenesis.Cells. 2022 Oct 1;11(19):3093. doi: 10.3390/cells11193093. Cells. 2022. PMID: 36231055 Free PMC article. Review.
References
-
- Adusumilli V. S., Walker T. L., Overall R. W., Klatt G. M., Zeidan S. A., Zocher S., et al. (2021). ROS dynamics delineate functional states of hippocampal neural stem cells and link to their activity-dependent exit from quiescence. Cell Stem Cell 28 300–314e6. 10.1016/j.stem.2020.10.019 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

