Role of Polyamines and Hypusine in β Cells and Diabetes Pathogenesis
- PMID: 35448531
- PMCID: PMC9028953
- DOI: 10.3390/metabo12040344
Role of Polyamines and Hypusine in β Cells and Diabetes Pathogenesis
Abstract
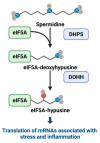
The polyamines-putrescine, spermidine, and spermine-are polycationic, low molecular weight amines with cellular functions primarily related to mRNA translation and cell proliferation. Polyamines partly exert their effects via the hypusine pathway, wherein the polyamine spermidine provides the aminobutyl moiety to allow posttranslational modification of the translation factor eIF5A with the rare amino acid hypusine (hydroxy putrescine lysine). The "hypusinated" eIF5A (eIF5Ahyp) is considered to be the active form of the translation factor necessary for the translation of mRNAs associated with stress and inflammation. Recently, it has been demonstrated that activity of the polyamines-hypusine circuit in insulin-producing islet β cells contributes to diabetes pathogenesis under conditions of inflammation. Elevated levels of polyamines are reported in both exocrine and endocrine cells of the pancreas, which may contribute to endoplasmic reticulum stress, oxidative stress, inflammatory response, and autophagy. In this review, we have summarized the existing research on polyamine-hypusine metabolism in the context of β-cell function and diabetes pathogenesis.
Keywords: diabetes; eIF5A; hypusine; polyamines; putrescine; spermidine; spermine; β cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hypusine, a polyamine-derived amino acid critical for eukaryotic translation.J Biol Chem. 2018 Nov 30;293(48):18710-18718. doi: 10.1074/jbc.TM118.003341. Epub 2018 Sep 26. J Biol Chem. 2018. PMID: 30257869 Free PMC article. Review.
-
Differential Mechanisms for the Involvement of Polyamines and Hypusinated eIF5A in Ebola Virus Gene Expression.J Virol. 2018 Sep 26;92(20):e01260-18. doi: 10.1128/JVI.01260-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045993 Free PMC article.
-
Inactivation of eukaryotic initiation factor 5A (eIF5A) by specific acetylation of its hypusine residue by spermidine/spermine acetyltransferase 1 (SSAT1).Biochem J. 2011 Jan 1;433(1):205-13. doi: 10.1042/BJ20101322. Biochem J. 2011. PMID: 20942800 Free PMC article.
-
The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A).J Biochem. 2006 Feb;139(2):161-9. doi: 10.1093/jb/mvj034. J Biochem. 2006. PMID: 16452303 Free PMC article. Review.
-
Synergistic drug combination GC7/DFMO suppresses hypusine/spermidine-dependent eIF5A activation and induces apoptotic cell death in neuroblastoma.Biochem J. 2018 Jan 31;475(2):531-545. doi: 10.1042/BCJ20170597. Biochem J. 2018. PMID: 29295892
Cited by
-
Structural Insights into the Mechanisms Underlying Polyaminopathies.Int J Mol Sci. 2024 Jun 7;25(12):6340. doi: 10.3390/ijms25126340. Int J Mol Sci. 2024. PMID: 38928047 Free PMC article. Review.
-
Inside the β Cell: Molecular Stress Response Pathways in Diabetes Pathogenesis.Endocrinology. 2022 Nov 14;164(1):bqac184. doi: 10.1210/endocr/bqac184. Endocrinology. 2022. PMID: 36317483 Free PMC article. Review.
-
The first evidence of biological activity for free Hypusine, an enigmatic amino acid discovered in the '70s.Amino Acids. 2023 Jul;55(7):913-929. doi: 10.1007/s00726-023-03283-4. Epub 2023 May 31. Amino Acids. 2023. PMID: 37258638
-
Stress and human health in diabetes: A report from the 19th Chicago Biomedical Consortium symposium.J Clin Transl Sci. 2023 Nov 20;7(1):e263. doi: 10.1017/cts.2023.646. eCollection 2023. J Clin Transl Sci. 2023. PMID: 38229904 Free PMC article.
-
Impact of dietary interventions on pre-diabetic oral and gut microbiome, metabolites and cytokines.Nat Commun. 2023 Sep 4;14(1):5384. doi: 10.1038/s41467-023-41042-x. Nat Commun. 2023. PMID: 37666816 Free PMC article. Clinical Trial.
References
-
- Burgos-Morón E., Abad-Jiménez Z., Martínez de Marañón A., Iannantuoni F., Escribano-López I., López-Domènech S., Salom C., Jover A., Mora V., Roldan I., et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019;8:1385. doi: 10.3390/jcm8091385. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

