A Systematic Review Assessing the Underrepresentation of Cancer Patients in COVID-19 Trials
- PMID: 35447626
- PMCID: PMC9254295
- DOI: 10.1159/000524623
A Systematic Review Assessing the Underrepresentation of Cancer Patients in COVID-19 Trials
Abstract
Background: The new severe acute respiratory syndrome coronavirus 2 has emerged as a global pandemic that threatens thousands around the world. Observational cohort studies have demonstrated that cancer patients have inferior outcomes due to underlying malignancy, treatment-related immunosuppression, or increased comorbidities. We aimed to examine the representation of cancer patients (hematological malignancies and solid tumors) in COVID-19 therapeutic and prophylactic interventional trials.
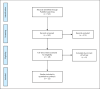
Methods: In this review, all randomized controlled trials (RCTs) published between December 2019 and August 2021 were included. We included only trials evaluating medications that were recommended by NIH guidelines: steroids, tocilizumab, remdesivir, and REGN-COV2.
Results: The search yielded 541 potentially relevant RCTs, 22 of which were considered suitable. All trials included patients with solid cancer and hematological malignancies in the formal reported inclusion criteria. However, only two trials reported the accurate number of cancer patients included. Ten trials excluded neutropenic patients and seven trials excluded thrombocytopenic patients. Eleven trials excluded patients that were treated with any immunosuppression treatment. None of the two trials that included cancer patients reported separate outcomes for this population.
Conclusion: Our systematic review shows that cancer patients are underrepresented in COVID-19 interventional therapeutic trials, and evidence regarding outcomes are lacking.
Keywords: Cancer; Coronavirus disease 2019; Severe acute respiratory syndrome coronavirus 2.
© 2022 S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
REGN-COV2 antibody cocktail in patients with SARS-CoV-2: Observational study from a single institution in Japan.J Infect Chemother. 2022 Jul;28(7):943-947. doi: 10.1016/j.jiac.2022.03.029. Epub 2022 Apr 7. J Infect Chemother. 2022. PMID: 35414436 Free PMC article.
-
A systematic review assessing the under-representation of elderly adults in COVID-19 trials.BMC Geriatr. 2020 Dec 20;20(1):538. doi: 10.1186/s12877-020-01954-5. BMC Geriatr. 2020. PMID: 33342426 Free PMC article.
-
A prospective, randomised, double blind placebo-controlled trial to evaluate the efficacy and safety of tocilizumab in patients with severe COVID-19 pneumonia (TOC-COVID): A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):470. doi: 10.1186/s13063-020-04447-3. Trials. 2020. PMID: 32493514 Free PMC article.
-
Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review.Rev Recent Clin Trials. 2021;16(2):138-145. doi: 10.2174/1574887115666200917110954. Rev Recent Clin Trials. 2021. PMID: 32940187 Review.
-
Comorbidities and mortality rate in COVID-19 patients with hematological malignancies: A systematic review and meta-analysis.J Clin Lab Anal. 2022 May;36(5):e24387. doi: 10.1002/jcla.24387. Epub 2022 Apr 6. J Clin Lab Anal. 2022. PMID: 35385130 Free PMC article. Review.
References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Jul;323((13)):1239–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical