Favipiravir for Treatment of Outpatients With Asymptomatic or Uncomplicated Coronavirus Disease 2019: A Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial
- PMID: 35446944
- PMCID: PMC9047233
- DOI: 10.1093/cid/ciac312
Favipiravir for Treatment of Outpatients With Asymptomatic or Uncomplicated Coronavirus Disease 2019: A Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial
Abstract
Background: Favipiravir, an oral, RNA-dependent RNA polymerase inhibitor, has in vitro activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Despite limited data, favipiravir is administered to patients with coronavirus disease 2019 (COVID-19) in several countries.
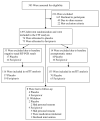
Methods: We conducted a phase 2, double-blind, randomized controlled outpatient trial of favipiravir in asymptomatic or mildly symptomatic adults with a positive SARS-CoV-2 reverse-transcription polymerase chain reaction assay (RT-PCR) within 72 hours of enrollment. Participants were randomized to receive placebo or favipiravir (1800 mg twice daily [BID] day 1, 800 mg BID days 2-10). The primary outcome was SARS-CoV-2 shedding cessation in a modified intention-to-treat (mITT) cohort of participants with positive enrollment RT-PCRs. Using SARS-CoV-2 amplicon-based sequencing, we assessed favipiravir's impact on mutagenesis.
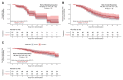
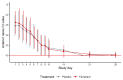
Results: We randomized 149 participants with 116 included in the mITT cohort. The participants' mean age was 43 years (standard deviation, 12.5 years) and 57 (49%) were women. We found no difference in time to shedding cessation overall (hazard ratio [HR], 0.76 favoring placebo [95% confidence interval {CI}, .48-1.20]) or in subgroups (age, sex, high-risk comorbidities, seropositivity, or symptom duration at enrollment). We detected no difference in time to symptom resolution (initial: HR, 0.84 [95% CI, .54-1.29]; sustained: HR, 0.87 [95% CI, .52-1.45]) and no difference in transition mutation accumulation in the viral genome during treatment.
Conclusions: Our data do not support favipiravir at commonly used doses in outpatients with uncomplicated COVID-19. Further research is needed to ascertain if higher favipiravir doses are effective and safe for patients with COVID-19.
Clinical trials registration: NCT04346628.
Keywords: COVID-19; SARS-CoV-2; clinical trial; favipiravir.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. H. reports payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from UpToDate. A. S. reports grants from Gilead Sciences, Regeneron Pharma, and Janssen Pharma. H. H. reports salary support from anonymous donors to Stanford University and grant support from the NIH (UL1 TR003142). C. E. reports financial support for the present manuscript from Fujifilm Pharmaceuticals USA (payments made to author’s consulting LLC). P. J. reports research support from anonymous donors to Stanford University to support clinical work. R. H. K. is currently the Chief Medical Officer for AiPharma Global Holdings LLC; is an unpaid consultant to Fujifilm Toyama Chemical Co Ltd and the Anti Viral Drug Development Alliance; and reports support for attending conferences and stock options from AiPharma Global Holdings LLC. J. P. reports grants from Heluna Health (seroepidemiological studies of SARS-CoV-2), Gauss Surgical (testing of an antigen test for SARS-CoV-2) and Ono Pharmaceuticals (clinical trial of Camostat for SARS-CoV-2). J. R. A. reports research support from anonymous donors to Stanford University. C. K. reports licenses from Clear Creek Bio and a patent assigned to Stanford University (“Use of a dihydroorotate dehydrogenase (DHODH) inhibitor in combination with an inhibitor of pyrimidine salvage,” US patent number 10,736,911; 2020). Y. M. reports grants from the NIH (U54 MD010724, U54 MD010724-05S1, R21AI148810, P30AG059307, 000522211-022), Pfizer (C3671008, C4591007), the Bill & Melinda Gates Foundation (OPP1113682), and the Chan Zuckerberg Foundation (12089sc); has received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from the American Academy of Pediatrics for the National Conference; and is on a Pfizer DSMB (non–COVID-19 vaccine trial). All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Similar articles
-
Early antiviral treatment in outpatients with COVID-19 (FLARE): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Mar 8;22(1):193. doi: 10.1186/s13063-021-05139-2. Trials. 2021. PMID: 33685502 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19.PLoS Med. 2022 Oct 19;19(10):e1004120. doi: 10.1371/journal.pmed.1004120. eCollection 2022 Oct. PLoS Med. 2022. PMID: 36260627 Free PMC article. Clinical Trial.
-
Antiviral therapy for COVID-19: Derivation of optimal strategy based on past antiviral and favipiravir experiences.Pharmacol Ther. 2022 Jul;235:108121. doi: 10.1016/j.pharmthera.2022.108121. Epub 2022 Feb 1. Pharmacol Ther. 2022. PMID: 35121001 Free PMC article. Review.
-
Understanding the clinical utility of favipiravir (T-705) in coronavirus disease of 2019: a review.Ther Adv Infect Dis. 2021 Dec 4;8:20499361211063016. doi: 10.1177/20499361211063016. eCollection 2021 Jan-Dec. Ther Adv Infect Dis. 2021. PMID: 34881025 Free PMC article. Review.
Cited by
-
A Clinical Insight on New Discovered Molecules and Repurposed Drugs for the Treatment of COVID-19.Vaccines (Basel). 2023 Feb 1;11(2):332. doi: 10.3390/vaccines11020332. Vaccines (Basel). 2023. PMID: 36851211 Free PMC article. Review.
-
Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial.BMC Infect Dis. 2024 Jan 15;24(1):89. doi: 10.1186/s12879-023-08835-3. BMC Infect Dis. 2024. PMID: 38225598 Free PMC article. Clinical Trial.
-
Clinical development of antivirals against SARS-CoV-2 and its variants.Curr Res Microb Sci. 2023 Dec 2;6:100208. doi: 10.1016/j.crmicr.2023.100208. eCollection 2024. Curr Res Microb Sci. 2023. PMID: 38149085 Free PMC article. Review.
-
Comparative Preclinical Pharmacokinetics and Disposition of Favipiravir Following Pulmonary and Oral Administration as Potential Adjunct Therapy Against Airborne RNA Viruses.Pharm Res. 2024 Nov;41(11):2189-2198. doi: 10.1007/s11095-024-03782-3. Epub 2024 Oct 17. Pharm Res. 2024. PMID: 39419926
-
Favipiravir for symptomatic COVID-19: A nationwide observational cohort study.J Infect Chemother. 2023 Feb;29(2):150-156. doi: 10.1016/j.jiac.2022.10.008. Epub 2022 Oct 26. J Infect Chemother. 2023. PMID: 36307058 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

