Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening
- PMID: 35443557
- PMCID: PMC9304440
- DOI: 10.5468/ogs.22017
Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening
Abstract
Objective: The objective of this study was to compare and evaluate the diagnostic value of serum carbohydrate antigen 125 (CA125) and/or human epididymis protein 4 (HE4) and a panel of novel multiple biomarkers in patients with ovarian tumors to identify more accurate and effective markers for screening ovarian cancer.
Methods: Candidate ovarian cancer biomarkers were selected based on a literature search. Dozens of candidate biomarkers were examined using 143 serum samples from patients with ovarian cancer and 157 healthy serum samples as noncancer controls. To select the optimal marker panel for an ovarian cancer classification model, a set of biomarker panels was created with the number of possible combinations of eight biomarkers. Using the set of biomarkers as an input variable, the optimal biomarker panel was selected by examining the performance of the biomarker panel set using the Random Forest algorithm as a non-linear classification method and a 10-fold cross-validation technique.
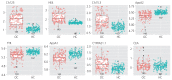
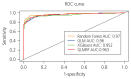
Results: The final selected optimal combination of five biomarkers (CA125, HE4, cancer antigen 15-3, apolipoprotein [Apo] A1, and ApoA2) exhibited a sensitivity of 93.71% and specificity of 93.63% for ovarian cancer detection during validation.
Conclusion: Combining multiple biomarkers is a valid strategy for ovarian cancer diagnosis and can be used as a minimally invasive screening method for early ovarian cancer. A panel of five optimal biomarkers, including CA125 and HE4, was verified in this study. These can potentially be used as clinical biomarkers for early detection of ovarian cancer.
Keywords: Algorithm; Biomarkers; Ovarian cancer; Screening.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
A multiplex biomarker assay improves the diagnostic performance of HE4 and CA125 in ovarian tumor patients.PLoS One. 2020 Oct 19;15(10):e0240418. doi: 10.1371/journal.pone.0240418. eCollection 2020. PLoS One. 2020. PMID: 33075095 Free PMC article.
-
Validation of a Biomarker Panel and Longitudinal Biomarker Performance for Early Detection of Ovarian Cancer.Int J Gynecol Cancer. 2016 Jul;26(6):1070-7. doi: 10.1097/IGC.0000000000000737. Int J Gynecol Cancer. 2016. PMID: 27206285 Free PMC article.
-
Clinical analysis of four serum tumor markers in 458 patients with ovarian tumors: diagnostic value of the combined use of HE4, CA125, CA19-9, and CEA in ovarian tumors.Cancer Manag Res. 2018 May 22;10:1313-1318. doi: 10.2147/CMAR.S155693. eCollection 2018. Cancer Manag Res. 2018. PMID: 29861641 Free PMC article.
-
[Ovarian tumor markers of presumed benign ovarian tumors].J Gynecol Obstet Biol Reprod (Paris). 2013 Dec;42(8):752-9. doi: 10.1016/j.jgyn.2013.09.030. Epub 2013 Nov 7. J Gynecol Obstet Biol Reprod (Paris). 2013. PMID: 24210243 Review. French.
-
Biomarkers in Ovarian Pathology: From Screening to Diagnosis. Review of the Literature.J Pers Med. 2021 Oct 29;11(11):1115. doi: 10.3390/jpm11111115. J Pers Med. 2021. PMID: 34834467 Free PMC article. Review.
Cited by
-
Some Aspects and Convergence of Human and Veterinary Drug Repositioning.Molecules. 2024 Sep 20;29(18):4475. doi: 10.3390/molecules29184475. Molecules. 2024. PMID: 39339469 Free PMC article. Review.
-
A personalized probabilistic approach to ovarian cancer diagnostics.Gynecol Oncol. 2024 Mar;182:168-175. doi: 10.1016/j.ygyno.2023.12.030. Epub 2024 Jan 23. Gynecol Oncol. 2024. PMID: 38266403 Free PMC article.
-
Combining TNFR2-Expressing Tregs and IL-6 as Superior Diagnostic Biomarkers for High-Grade Serous Ovarian Cancer Masses.Cancers (Basel). 2023 Jan 21;15(3):667. doi: 10.3390/cancers15030667. Cancers (Basel). 2023. PMID: 36765633 Free PMC article.
-
Comparison of International Ovarian Tumor Analysis ADNEX model and Ovarian-Adnexal Reporting and Data System with final histological diagnosis in adnexal masses: a retrospective study.Obstet Gynecol Sci. 2024 Jan;67(1):86-93. doi: 10.5468/ogs.23061. Epub 2023 Oct 12. Obstet Gynecol Sci. 2024. PMID: 37822234 Free PMC article.
-
Proteomics and Bioinformatics Investigations Link Overexpression of FGF8 and Associated Hub Genes to the Progression of Ovarian Cancer and Poor Prognosis.Biochem Res Int. 2024 Sep 13;2024:4288753. doi: 10.1155/2024/4288753. eCollection 2024. Biochem Res Int. 2024. PMID: 39309198 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95 (Suppl 1):S161–92. - PubMed
-
- Brun JL, Fritel X, Levêque J. Clinical practice guidelines: presumed benign ovarian tumors--aims, methods, and organization. J Gynecol Obstet Biol Reprod (Paris) 2013;42:710–4. - PubMed
-
- Vaisbuch E, Dgani R, Ben-Arie A, Hagay Z. The role of laparoscopy in ovarian tumors of low malignant potential and early-stage ovarian cancer. Obstet Gynecol Surv. 2005;60:326–30. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

