Elevated tumor markers for monitoring tumor response to immunotherapy
- PMID: 35434583
- PMCID: PMC9011015
- DOI: 10.1016/j.eclinm.2022.101381
Elevated tumor markers for monitoring tumor response to immunotherapy
Abstract
Background: As the immune-related response evaluation criteria in solid tumors (irRECIST) by imaging greatly underestimated the objective response to immunotherapy, we established the response evaluation criteria in solid tumors based on tumor markers (RecistTM) to explore whether RecistTM can compensate for the deficiencies of the irRECIST criteria.
Methods: This was an observational study, which consisted of two parts. The first part (Group A) was a retrospective study including the patients with malignant solid tumors. The second part (Group B) was a prospective study, which were EGFR-negative and ALK-negative patients with stage IIIB-IV non-small cell lung cancer receiving first-line treatment. From January 2017 to September 2020, one hundred and ten patients with a three-time increase in tumor markers receiving immunotherapy were recruited. The treatment response to immunotherapy was evaluated by irRECIST and RecistTM. Efficacy, overall survival (OS), first evaluation time and earliest response time under the different evaluation criteria were compared by statistics.
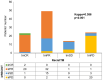
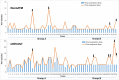
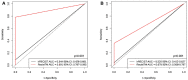
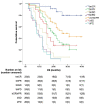
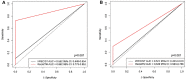
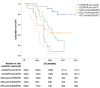
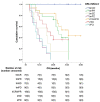
Findings: The treatment response evaluated by the RecistTM criteria was not consistent with that evaluated by the irRECIST criteria (Kappa = 0.386, p < 0.001). RecistTM had a higher completed response (CR) rate compared to irRECIST criteria (20.9% vs 1.8%, p < 0.001). The earliest response time under the RecistTM criteria was 3.42 weeks earlier than that under the irRECIST criteria (u = -5.233, p < 0.001). There were significant differences in median OS between tumor marker-related complete response (tmCR) and tumor marker-related partial response (tmPR), as well as between tmPR and tumor marker-related stable disease (tmSD) (χ2 = 15.572, p < 0.001; χ2 = 7.720, p = 0.005), but not between tmSD and tumor marker-related progressive disease (tmPD) (χ2 = 1.596, p = 0.206). When applying both criteria together, for patients with immune-related CR / immune-related PR (irCR/irPR) (n = 54) under irRECIST criteria, there was a significant difference in median OS between achieving tmCR (n = 22) and tmPR (n = 32) (χ2 = 14.011, p < 0.001). RecistTM criteria can predict 1-year and 2-year OS more accurately than irRECIST criteria (AUCs:0.862 vs 0.552, 0.649 vs 0.521, respectively;both p < 0.001). In RecistTM, 4 patients had been observed with pseudoprogression in tumor markers.
Interpretation: The RecistTM criteria could effectively distinguish CR, PR, and SD, which may help resolve the shortcomings of the RECIST criteria in evaluating the treatment response to immunotherapy, especially in assessing whether patients can achieve deep or even complete response as soon as possible.
Funding: This work was supported by the Key projects of Chongqing Health and Family Planning Commission (to Xueqin Yang, 2019ZDXM011).
Keywords: CR, Complete response; Efficacy evaluation; ICIs, Immune checkpoint inhibitors; Immunotherapy; NE, Not estimated; NSCLC, Non-small cell lung cancer; ORR, Objective response rate; OS, Overall survival; PD, Progressive disease; PR, Partial response; RECIST; RECIST, Response Evaluation Criteria in Solid Tumors; RecistTM, Response evaluation criteria in solid tumors based on tumor markers; SD, Stable disease; Tumor markers; irCR, Immune-related complete response; irPD, Immune-related progression disease; irPR, Immune-related partial response; irRECIST, Immune-related Response Evaluation Criteria in Solid Tumors; irSD, Immune-related stable disease; tmCR, Tumor marker-related complete response; tmPD, Tumor marker-related progression disease; tmPR, Tumor marker-related partial response; tmSD, Tumor marker-related stable disease.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Tumor marker-based RecistTM is superior to RECIST as criteria to predict the long-term benefits of targeted therapy in advanced non-small-cell lung cancer with driver gene mutations.Neoplasia. 2024 Jul;53:101006. doi: 10.1016/j.neo.2024.101006. Epub 2024 May 17. Neoplasia. 2024. PMID: 38761505 Free PMC article.
-
Comparison of tumor assessments using RECIST 1.1 and irRECIST, and association with overall survival.J Immunother Cancer. 2022 Feb;10(2):e003302. doi: 10.1136/jitc-2021-003302. J Immunother Cancer. 2022. PMID: 35228264 Free PMC article.
-
Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria.Eur J Cancer. 2018 Jan;88:38-47. doi: 10.1016/j.ejca.2017.10.017. Epub 2017 Nov 26. Eur J Cancer. 2018. PMID: 29182990
-
Tumor response assessment on imaging following immunotherapy.Front Oncol. 2022 Oct 25;12:982983. doi: 10.3389/fonc.2022.982983. eCollection 2022. Front Oncol. 2022. PMID: 36387133 Free PMC article. Review.
-
Immunotherapy and the role of imaging.Cancer. 2018 Jul 15;124(14):2906-2922. doi: 10.1002/cncr.31349. Epub 2018 Apr 19. Cancer. 2018. PMID: 29671876 Review.
Cited by
-
Pseudoprogression of thoracic tumor after radiotherapy in the era of immunotherapy: a case series.Front Oncol. 2023 Jul 28;13:1021253. doi: 10.3389/fonc.2023.1021253. eCollection 2023. Front Oncol. 2023. PMID: 37576884 Free PMC article.
-
Cell-free multi-omics analysis reveals potential biomarkers in gastrointestinal cancer patients' blood.Cell Rep Med. 2023 Nov 21;4(11):101281. doi: 10.1016/j.xcrm.2023.101281. Cell Rep Med. 2023. PMID: 37992683 Free PMC article.
-
Tumor marker-based RecistTM is superior to RECIST as criteria to predict the long-term benefits of targeted therapy in advanced non-small-cell lung cancer with driver gene mutations.Neoplasia. 2024 Jul;53:101006. doi: 10.1016/j.neo.2024.101006. Epub 2024 May 17. Neoplasia. 2024. PMID: 38761505 Free PMC article.
References
-
- Ferté C., Marabelle A. iRECIST: a clarification of tumour response assessment in the immunotherapy era. Eur J Cancer. 2017;77:165–167. - PubMed
-
- Billan S., Kaidar-Person O., Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020;21:e463–e476. - PubMed
-
- Borcoman E., Kanjanapan Y., Champiat S., et al. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30:385–396. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

