Therapeutic advantages of combined gene/cell therapy strategies in a murine model of GM2 gangliosidosis
- PMID: 35434178
- PMCID: PMC8983315
- DOI: 10.1016/j.omtm.2022.03.011
Therapeutic advantages of combined gene/cell therapy strategies in a murine model of GM2 gangliosidosis
Abstract
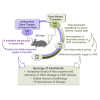
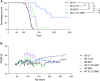
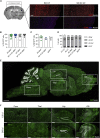
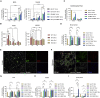
Genetic deficiency of β-N-acetylhexosaminidase (Hex) functionality leads to accumulation of GM2 ganglioside in Tay-Sachs disease and Sandhoff disease (SD), which presently lack approved therapies. Current experimental gene therapy (GT) approaches with adeno-associated viral vectors (AAVs) still pose safety and efficacy issues, supporting the search for alternative therapeutic strategies. Here we leveraged the lentiviral vector (LV)-mediated intracerebral (IC) GT platform to deliver Hex genes to the CNS and combined this strategy with bone marrow transplantation (BMT) to provide a timely, pervasive, and long-lasting source of the Hex enzyme in the CNS and periphery of SD mice. Combined therapy outperformed individual treatments in terms of lifespan extension and normalization of the neuroinflammatory/neurodegenerative phenotypes of SD mice. These benefits correlated with a time-dependent increase in Hex activity and a remarkable reduction in GM2 storage in brain tissues that single treatments failed to achieve. Our results highlight the synergic mode of action of LV-mediated IC GT and BMT, clarify the contribution of treatments to the therapeutic outcome, and inform on the realistic threshold of corrective enzymatic activity. These results have important implications for interpretation of ongoing experimental therapies and for design of more effective treatment strategies for GM2 gangliosidosis.
Keywords: CNS; GM2 gangliosidosis; Sandhoff disease; bone marrow transplantation; cell therapy; gene therapy; hexosaminidase; lentiviral vectors; lysosomal storage disorders.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Novel bicistronic lentiviral vectors correct β-Hexosaminidase deficiency in neural and hematopoietic stem cells and progeny: implications for in vivo and ex vivo gene therapy of GM2 gangliosidosis.Neurobiol Dis. 2020 Feb;134:104667. doi: 10.1016/j.nbd.2019.104667. Epub 2019 Nov 1. Neurobiol Dis. 2020. PMID: 31682993
-
Treatment of GM2 Gangliosidosis in Adult Sandhoff Mice Using an Intravenous Self-Complementary Hexosaminidase Vector.Curr Gene Ther. 2022;22(3):262-276. doi: 10.2174/1566523221666210916153051. Curr Gene Ther. 2022. PMID: 34530708
-
Systemic Gene Transfer of a Hexosaminidase Variant Using an scAAV9.47 Vector Corrects GM2 Gangliosidosis in Sandhoff Mice.Hum Gene Ther. 2016 Jul;27(7):497-508. doi: 10.1089/hum.2016.015. Hum Gene Ther. 2016. PMID: 27199088
-
[Molecular pathogenesis and therapeutic approach of GM2 gangliosidosis].Yakugaku Zasshi. 2013;133(2):269-74. doi: 10.1248/yakushi.12-00199. Yakugaku Zasshi. 2013. PMID: 23370522 Review. Japanese.
-
[Recent advances in molecular genetics of GM2 gangliosidosis].Nihon Rinsho. 1995 Dec;53(12):2988-93. Nihon Rinsho. 1995. PMID: 8577047 Review. Japanese.
Cited by
-
Gene and Cellular Therapies for Leukodystrophies.Pharmaceutics. 2023 Oct 24;15(11):2522. doi: 10.3390/pharmaceutics15112522. Pharmaceutics. 2023. PMID: 38004502 Free PMC article. Review.
-
CRISPR/nCas9-Based Genome Editing on GM2 Gangliosidoses Fibroblasts via Non-Viral Vectors.Int J Mol Sci. 2022 Sep 14;23(18):10672. doi: 10.3390/ijms231810672. Int J Mol Sci. 2022. PMID: 36142595 Free PMC article.
References
-
- Mahuran D.J. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim. Biophys. Acta. 1999;1455:105–138. - PubMed
-
- Cordeiro P., Hechtman P., Kaplan F. The GM2 gangliosidoses databases: allelic variation at the HEXA, HEXB, and GM2A gene loci. Genet. Med. 2000;2:319–327. - PubMed
-
- Tsuji D., Akeboshi H., Matsuoka K., Yasuoka H., Miyasaki E., Kasahara Y., Kawashima I., Chiba Y., Jigami Y., Taki T., et al. Highly phosphomannosylated enzyme replacement therapy for GM2 gangliosidosis. Ann. Neurol. 2011;69:691–701. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

